Question bank
Chapter-1 Matter
Q-1
Images A and B show phenomena in which water is formed. Answer the following questions.
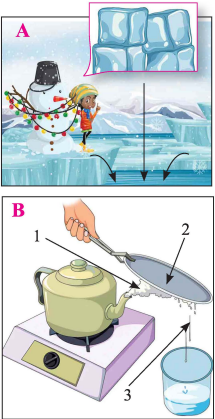
(i) Name the process by which water is formed in A and in B.
(ii) B is observed in nature as a part of the water cycle.
a. State the equivalent of parts numbered 1, 2 and 3 in the water cycle.
b. State one major difference between the process 1 shown in B and that taking place in the water cycle.
(iii) If a change of state requires heat, the body takes up the heat from the surroundings and the environment cools down. If the change requires to be cooled, then the body gives up its heat to the surroundings in order to cool down, and the environment become warm .
State which of the following process will cool the environment:
a. Process shown in image A.
b. Process taking place at 3 in image B.
c. Process taking place at 2 in image B.
Q-2 Define the following terms and answer the associated questions:
Q-3 Name the phenomenon that causes the following occurrences:
(a) Rainfall.
Q-4 Answer the following Questions:
(a) Distinguish between gas and vapour.
(b) Distinguish between boiling and evaporation. Also give one common example of each.
Q-5 State whether the following statements are true or false :
Multiple Choice Questions
Q-1 Solids do not diffuse because:
(i)
They have many free surfaces(ii)
They lack intermolecular spaces(iii)
They have a high density(iv)
None of these
Q-2 Carbon dioxide can be liquefied by:
(i)
Increasing the temperature(ii)
Compressing the gas and suddenly releasing the pressure(iii)
Decreasing the pressure of the gas(iv)
All of the above
Q-3 Which statement is True for the molecular structure of solids?
(i)
Atoms vibrate about their mean position(ii)
Intermolecular space is negligible(iii)
lntramolecular attraction is strong(iv)
All statements are true
Q-4 When a solid is heated:
(i)
Its particles leave their position(ii)
It becomes fluid(iii)
Intermolecular space increases(iv)
All of these
Q-5 Which of the following is NOT a characteristic of gases?
(i)
Loose, random arrangement of particles(ii)
Highly compressible(iii)
Mingle with particles of other gases(iv)
Very rigid
Q-6 Which of the following statement is False for gases?
(i)
Strong attractive force between particles(ii)
Large gaps between particles(iii)
Can be stored only in closed containers(iv)
No fixed shape and volume
Q-7 Diffusion is the movement of particles from their region of:
(i)
Higher to lower concentration(ii)
Lower to higher concentration(iii)
Both a and b are correct(iv)
None of these
Q-8 Which of the following is True for matter?
(i)
Matter expands when cooled(ii)
Matter contracts when cooled(iii)
Matter expands when heated(iv)
b and c are true
Q-9 The conversion of matter from solid to gaseous state is called:
(i)
Condensation(ii)
Sublimation(iii)
Deposition(iv)
Evaporation
Q-10 When ice melts, heat is _____________ :
(i)
Given out(ii)
Taken in(iii)
Unchanged(iv)
None of these
Q-11 Water droplets are seen on the surface of the bottle taken out from the fridge due to:
(i)
Condensation(ii)
Deposition(iii)
Evaporation(iv)
None of these
Q-12 When water is cooled, the motion of its particles become:
(i)
Slow(ii)
Fast(iii)
Unchanged(iv)
None of these
Q-13 Which of the following can be described as being 'fluid'?
(i)
Solids(ii)
Liquids(iii)
Gases(iv)
Both band c
Q-14 In water, the molecules pull together into a spherical shape due to the force of:
(i)
Gravity(ii)
Cohesion(iii)
Adhesion(iv)
Magnetism
Q-15 The meniscus of mercury is:
(i)
Concave(ii)
Convex(iii)
Horizontal(iv)
None of theseChapter-2 Physical and Chemical Change
Q-1 State whether the statements refer to a physical change or a chemical change:
(e) This type of change can be easily reversed.
Q-2 Classify the following as physical or chemical change. Give a reason for your answer.
Q-3 Give one example of a chemical reaction in which:
(d) Sound is produced.
Q-4 Answer the following Questions:
Q-5 The image shows a process that takes place in green plants. Given below is a list of terms that describe the various types of changes that occur around us.
Natural change, Chemical change, Physical change, Periodic change, Slow change, Fast change, Reversible change, Irreversible change, Temporary change, Permanent Change
(c) In this reaction light energy is absorbed . Hence this can be described as a/an ______________ reaction.
Multiple Choice Questions
Q-1 Which of the following is a characteristic of a physical change?
(i)
It can be reversed easily(ii)
It is a permanent change(iii)
New substances are formed(iv)
None of these
Q-2 Growth of a crystal is an example of:
(i)
Change of state(ii)
Irreversible change(iii)
Physical change(iv)
Chemical change
Q-3 Changes that affect properties like shape, size, state and colour are called:
(i)
Physical change(ii)
Periodic change(iii)
Natural change(iv)
All of these
Q-4 When salt dissolves in water, it loses its:
(i)
Salty taste(ii)
Crystalline shape(iii)
Neither a nor b(iv)
All of these
Q-5 Which of the following is a physical change?
(i)
Change of state(ii)
Photosynthesis(iii)
Respiration(iv)
All of these
Q-6 When sulphur is heated with iron the change is chemical because:
(i)
A new substance is formed(ii)
Heat and light is given out(iii)
The change is permanent(iv)
All of these
Q-7 The burning of magnesium is:
(i)
A fast change(ii)
Chemical change(iii)
Exothermic(iv)
All of these
Q-8 The daily rise and fall of tides is a:
(i)
Periodic change(ii)
Non-periodic change(iii)
Natural change(iv)
Both a and c are true
Q-9 Action of heat on copper carbonate is a chemical change because:
(i)
A gas carbon dioxide is evolved(ii)
A new black substance is formed(iii)
The change is permanent(iv)
All of these
Q-10 When a magnesium ribbon burns in oxygen it gives out:
(i)
Heat(ii)
Light(iii)
Sound(iv)
Only a and b
Q-11 Water can be decomposed by passing _____________ through it.
(i)
Light(ii)
Electricity(iii)
Neither(iv)
Both
Q-12 An example of a photochemical reaction is:
(i)
Respiration(ii)
Electrolysis(iii)
Photosynthesis(iv)
All of these
Q-13 Which gas burns with a 'pop' sound?
(i)
Oxygen(ii)
Nitrogen(iii)
Hydrogen(iv)
Carbon dioxide
Q-14 When sodium is added to water, the gas evolved is:
(i)
Oxygen(ii)
Nitrogen(iii)
Hydrogen(iv)
Carbon dioxide
Q-15 When water freezes into ice, the density of ice is _______________ that of water:
(i)
Less than(ii)
More than(iii)
Same as(iv)
None of theseChapter-3 Elements, Compounds and Mixtures
Q-1 Define the following terms:
Q-2 Classify the following under elements, compounds, mixtures:
Q-3 Name the technique that you would use to separate the following mixtures:
Q-4 Show with a flowchart how the following separations can be carried out:
Q-5 Differentiate between mixtures and compounds on the basis of:
Q-6 State the principle underlying the following separation techniques:
Multiple Choice Questions
Q-1 Which of the following is a liquid metal?
(i)
Gallium(ii)
Mercury(iii)
Francium(iv)
All of these
Q-2 There are ______________ gaseous elements known to us.
(i)
9(ii)
10(iii)
11(iv)
12
Q-3 There are _____________ liquid elements known to us.
(i)
6(ii)
3(iii)
4(iv)
2
Q-4 Which of the following is NOT a homogeneous mixture?
(i)
Alcohol + water(ii)
Gun powder(iii)
Ammonia + hydrogen(iv)
Sulphur + carbon disulphide
Q-5 Which of the following is a homogeneous mixture?
(i)
Petrol + kerosene(ii)
Petrol + water(iii)
Oil + water(iv)
Sulphur trioxide + water
Q-6 Chromatography can be used to separate pigments of:
(i)
Ink(ii)
Flower(iii)
Chlorophyll(iv)
All of these
Q-7 The following elements show polyatomicity:
(i)
Sulphur(ii)
Boron(iii)
Phosphorus(iv)
All of these
Q-8 There are _________________ elements known to us.
(i)
125(ii)
91(iii)
118(iv)
101
Q-9 The symbol of an element can be:
(i)
First letter of its English name(ii)
First two letters of its English name(iii)
Derived from its Latin name(iv)
All of these
Q-10 Which of the following element is triatomic?
(i)
Phosphorus(ii)
Argon(iii)
Ozone(iv)
Chlorine
Q-11 Which of the following statement is true?
(i)
Mixtures are always homogeneous(ii)
Water is a mixture(iii)
Air is a compound(iv)
Non-metals are elements
Q-12 Which statement is NOT true for compounds?
(i)
They are never homogeneous(ii)
Fixed composition by weight(iii)
Can be shown by a formula(iv)
Its formation involves energy change
Q-13 When iron and sulphur are mixed and heated together, the product formed is a:
(i)
Mixture(ii)
Compound(iii)
Element(iv)
None of these
Q-14 The water molecule is made up of _____________ atoms:
(i)
2(ii)
3(iii)
1(iv)
4Chapter-4 Atomic Structure
Q-1 Answer the following Questions:
(a) What does the word atom mean?
(b) State Dalton's atomic theory.
(c) How did Niels Bohr visualise the atom?
(d) Explain the statement 'An atom is electrically neutral'.
Q-2 Name the particle or particles you would expect to find in:
Q-3 An atom has 13 protons and 14 neutrons.
Q-4 A certain particle 'X' has 17 protons, 18 electrons and 20 neutrons.
Q-5 Fill in the blanks by selecting the correct term:
Multiple Choice Questions
Q-1 Which of the following belong to Group 3 of the periodic table?
(i)
2, 8, 1
(ii)
2, 3
(iii)
2, 8, 3
(iv)
Only band c
Q-2 Which of the following belong to Period 3 of the periodic table?
(i)
2, 8, 1
(ii)
2, 3
(iii)
2, 8, 3
(iv)
Only a and c
Q-3 Which statement is true for an atom with 3 valence electrons?
(i)
It is a metal
(ii)
It belongs to Period 3
(iii)
It belongs to Group 3
(iv)
Both a and c
Q-4 Which statement is true for an element with atomic number 7?
(i)
It is a non-metal
(ii)
It belongs to Period 7
(iii)
It belongs to Group 7
(iv)
It is a metal
Q-5 Which statement is true for an element with atomic number 8?
(i)
It is a noble gas
(ii)
It is a non-metal
(iii)
It belongs to Group 8
(iv)
It is a metal
Q-6 An atom having atomic number 12 will ______ 2 electrons to form a _______.
(i)
Lose, anion
(ii)
Lose, cation
(iii)
Gain, anion
(iv)
Gain, cation
Q-7 An atom having atomic number 10 will _______ electrons.
(i)
Lose electrons
(ii)
Gain electrons
(iii)
Neither lose nor gain
(iv)
None of these
Chapter-5 Language of Chemistry
Q-1
Write the chemical formula of the following compounds:
(a) Zinc sulphide
(b) Copper(II) oxide
(c) Sodium carbonate
(d) Potassium chlorate
(e) Ferrous nitrate
(f) Silver oxide
(g) Lead(II) sulphide
(h) lron(II) oxide
(i) Potassium carbonate
(j) Sodium chlorate
(k) Cobalt nitrate
(l) Silver bromide
(m) Zinc nitrate
(n) lron(III) oxide
(o) Calcium carbonate
Q-2
Name the following compounds:
(i) CuS
(ii) Kl
Chapter-6 Metals and Non-Metals
Q-1 Explain the following terms:
Q-2 Fill in the blanks:
Q-3 Name the type of protective coating that is usually applied to prevent corrosion in the following:
Q-4 Name:
Q-5 State one use of each:
Multiple Choice Questions
Q-1 Which is a non-reactive metal?
(i)
Iron(ii)
Magnesium(iii)
Gold(iv)
Aluminium
Q-2 Which is a liquid non-metal?
(i)
Mercury(ii)
Galium(iii)
Bromine(iv)
All of these
Q-3 Electronegativity is a property of:
(i)
Metals(ii)
Non-metals(iii)
Metalloids(iv)
Both a and c
Q-4 Which metal is found in the uncombined state in nature?
(i)
Gold(ii)
Silver(iii)
Platinum(iv)
All of these
Q-5 Iron rusts in the presence of:
(i)
Moisture(ii)
Oxygen(iii)
Carbon dioxide(iv)
All of these
Q-6 The tendency of a metal to lose elecrons to form positive ions is called:
(i)
Electronegativity(ii)
Electropositivity(iii)
Electron affinity(iv)
None of these
Q-7 This metal can react with acids as well as alkalis:
(i)
Copper(ii)
Aluminium(iii)
Iron(iv)
Sodium
Q-8 A metallic coating to prevent rusting of articles is:
(i)
Tin plating(ii)
Oil paint(iii)
Enamel(iv)
Grease
Q-9 A non-metallic coating to prevent rusting of articles is:
(i)
Galvanising(ii)
Enamel(iii)
Electroplating(iv)
Alloying
Q-10 Which of the following is an alloy?
(i)
Stainless steel(ii)
Brass(iii)
Bronze(iv)
All of these
Q-11 Which is a metalloid?
(i)
Silicon(ii)
Boron(iii)
Antimony(iv)
All of these
Q-12 Metals which react with acids and bases are called:
(i)
Metalloids(ii)
Amphoteric(iii)
Indicators(iv)
None of these
Q-13 The best conductor of electricity is:
(i)
Aluminium(ii)
Silver(iii)
Boron(iv)
Tungsten
Q-14 Metals with low melting point is:
(i)
Sodium(ii)
Mercury(iii)
Potassium(iv)
Both a and c
Q-15 This is a form of carbon:
(i)
Diamond(ii)
Graphite(iii)
Coal(iv)
All of theseChapter-7 Air, Oxygen and Oxides
Q-1 Answer the following Questions:
(a) Name the main components of air.
(b) State three uses of water vapour in the air.
(c) Give reasons to support the fact that air is a mixture.
(d) Explain: When a candle burns, it appears to lose weight.
(e) Name the products formed when a candle burns in air.
(f) An experiment was set up as shown below to show that a candle gains weight when it burns.
(i) What is A?
(ii) State the function of A.
(iii) What is B?
(iv) State the function of B.
(v) What is C?
(vi) State the function of C.
(g) Distinguish between air and oxygen.
Q-2 State one method by which:
Q-3 Write word equations to obtain oxygen from:
Q-4 The diagram below shows a method of preparing oxygen in the laboratory.
Q-5 Fill in the blanks:
Q-6 Complete the following reactions and state the type of oxide being prepared.
Multiple Choice Questions
Q-1 Which of the following gas is not present in air?
(i)
Nitrogen(ii)
Hydrogen(iii)
Oxygen(iv)
Carbon dioxide
Q-2 The 12 Day Experiment was performed by:
(i)
John Mayow(ii)
Robert Boyle(iii)
Antoine Lavoisier(iv)
Robert Hooke
Q-3 Carbon dioxide is:
(i)
Combustible but non supporter of combustion(ii)
Non-combustible and a non supporter of combustion(iii)
Non-combustible but supporter of combustion(iv)
None of these
Q-4 Presence of nitrogen in the air is necessary for:
(i)
Diluting the effect of oxygen(ii)
Carrying out photosynthesis(iii)
Providing plants with protein source(iv)
Only a and c are true
Q-5 Which of the following is an oxidation reaction?
(i)
Respiration(ii)
Rusting(iii)
Burning(iv)
All of these
Q-6 Which of the following is an amphoteric oxide?
(i)
Zinc oxide(ii)
Magnesium oxide(iii)
Calcium oxide(iv)
Nitric oxide
Q-7 Which of the following is a neutral oxide?
(i)
Zinc oxide(ii)
Magnesium oxide(iii)
Calcium oxide(iv)
Nitric oxide
Q-8 Which of the following is an air pollutant?
(i)
Carbon monoxide(ii)
Sulphur dioxide(iii)
Nitrogen dioxide(iv)
All of these
Q-9 Which feature is common to rusting, burning and respiration?
(i)
All use oxygen(ii)
All are rapid processes(iii)
All give out heat and light(iv)
All are slow processes
Q-10 Which of these can form both acidic as well as neutral oxides?
(i)
Sulphur(ii)
Carbon(iii)
Nitrogen(iv)
Only b and c
Q-11 Which of the following is a drying agent?
(i)
Cone. Sulphuric acid(ii)
Phosphorus pentoxide(iii)
Fused Calcium chloride(iv)
All of these
Q-12 Oxygen is collected by the downward displacement of water because it is:
(i)
Lighter than air(ii)
Heavier than air(iii)
Soluble in water(iv)
Insoluble in water
Q-13 The gases that pollute the air are generally the products of:
(i)
Combustion(ii)
Respiration(iii)
Photosynthesis(iv)
None of these
Q-14 Acid rain contains high levels of:
(i)
Carbonic acid(ii)
Sulphuric acid(iii)
Nitric acid(iv)
Both b and c
Q-15 Binary compounds of oxygen with metals and non-metals are called:
(i)
Metalloids(ii)
Alloys(iii)
Oxides(iv)
Ores