Question bank
Chapter-1 Introduction to Chemistry
Q-1
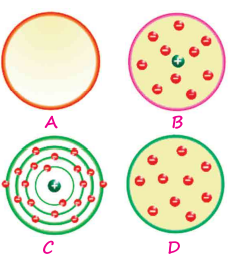
Four different models of the atom are shown - A, B, C and D. In the table given below, write the name assigned to each model, name of the scientist and the year in which the model was proposed.
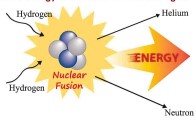
Q-2 The Sun is a huge, fiery ball of gaseous matter, the two most abundant gases being hydrogen (70%) and helium (28%). The hydrogen atoms, through a series of chemical reactions, fuse or combine to form helium atoms. This fusion reaction releases energy in the form of heat and light.
Q-3 Rocks and Minerals on the Earth's crust are a rich source of raw materials. Many metals can be extracted from the ores found in rocks.
Q-4
Cakes and pastries are decorated with colourful icing. Find out the names of two chemicals that are used as edible colouring matter.
Q-5 Many useful substances occur in nature. Raw materials from a variety of sources can be converted into new and useful products by chemical changes. For example:
(b) Green plants use carbon dioxide and water in the presence of sunlight (radiant energy) to prepare food in the form of carbohydrates (glucose)
Q-6 Fill in the blanks using only the terms Combustion, respiration, photosynthesis
Q-7 An experiment was set up as shown.
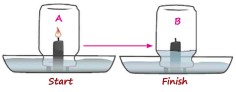
(b) Give an explanation for the changes mentioned in (i)
(e) If a leafy twig was placed in the jar along with the burning candle, and the apparatus is placed in sunlight, what are you likely to observe? Explain
Q-8 In the formation of rust, four atoms of iron combine with six atoms of oxygen.

(d) Is iron an element or a compound? Justify your answer.
(e) Name the first scientist who gave a scientific explanation of the atom.
Q-9 Answer the following questions.
Q-10 Name two natural fibres of:
Multiple Choice Questions
Q-1 This gas is not present in Earth's atmosphere:
(i)
Helium(ii)
Hydrogen(iii)
Nitrogen(iv)
Carbon dioxideQ-2 This is the most abundant element in the Earth's crust:
(i)
Copper(ii)
Gold(iii)
Aluminium(iv)
Iron
Q-3 Carbonates are metals combined with carbon and:
(i)
Oxygen(ii)
Nitrogen(iii)
Sulphur(iv)
Hydrogen
Q-4 This is not an element:
(i)
Oxygen(ii)
Nitrogen(iii)
Water(iv)
HydrogenQ-5 Metals that occur in rocks are called:
(i)
Alloys(ii)
Ores(iii)
Compounds(iv)
ElementsQ-6 This metal could be extracted only after electricity was discovered:
(i)
Gold(ii)
Iron(iii)
Sodium(iv)
CopperQ-7 The four element theory of matter was put forth by:
(i)
Socrates(ii)
Plato(iii)
Aristotle(iv)
Robert BoyleQ-8 Which of the following is NOT part of the scientific method?
(i)
Making a guess(ii)
Making an observation(iii)
Conducting an experiment(iv)
Forming a hypothesisQ-9 Which statement is NOT part of Dalton's postulates?
(i)
Atoms are divisible(ii)
All atoms of an element are identical.(iii)
Atoms are indivisible(iv)
Atoms cannot be created or destroyedQ-10 The Plum Pudding model of the atom was put forth by:
(i)
Ernest Rutherford(ii)
Niels Bohr(iii)
Robert Boyle(iv)
J.J Thomson
Q-11 Which of these is NOT a synthetic fibre:
(i)
Cotton(ii)
Nylon(iii)
Polyester(iv)
RayonQ-12 Which of these fibres is sourced from animals?
(i)
Cotton(ii)
Jute(iii)
Silk(iv)
None of theseQ-13 Which of these is NOT a fossil fuel?
(i)
Coal(ii)
Natural Gas(iii)
Petroleum(iv)
None of theseQ-14 Which of these is NOT a man-made material?
(i)
Plastic(ii)
Detergents(iii)
Paint and dyes(iv)
None of theseQ-15 Carbon dioxide was first prepared by:
(i)
John Mayow(ii)
Joseph Black(iii)
Lavoisier(iv)
Robert BoyleChapter-2 Fundamentals of Matter
Q-1 State whether the following statements are true or false:
Q-2 Give one term for the properties stated below. Also give one example for each property.
Q-3 Study the illustration given below and answer the questions that follow:
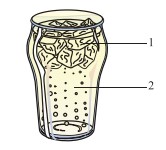
(c) Suggest a method by which the solid can be converted into a liquid.
(d) Suggest a method by which the liquid can be converted into a solid.
Q-4 Name:
Multiple Choice Questions
Q-1 Which of the following is NOT matter?
(i)
Spoon(ii)
Mercury(iii)
Heat(iv)
Carbon dioxideQ-2 Which of the following is NOT a subatomic particle?
(i)
Neutron(ii)
Proton(iii)
Nucleus(iv)
ElectronQ-3 Which of the following is NOT a characteristic of solids?
(i)
Rigid(ii)
Compressible(iii)
Fixed shape(iv)
Fixed volumeQ-4 Which of the following is NOT a characteristic of liquids?
(i)
Fluid(ii)
Fixed shape(iii)
Fixed volume(iv)
One free surfaceQ-5 Which of the following is NOT a characteristic of gases?
(i)
Loose, random arrangement of particles(ii)
Highly compressible(iii)
Mingle with particles of other gases(iv)
Very rigidQ-6 Which of the following statement is False for gases?
(i)
Strong attractive force between particles(ii)
Large gaps between particles(iii)
Can be stored only in closed containers(iv)
No fixed shape and volumeQ-7 Which of these is the effect of heat on matter?
(i)
Change in state(ii)
Change in size(iii)
Chemical change(iv)
All of theseQ-8 Which of the following is True for matter?
(i)
Matter expands when cooled(ii)
Matter contracts when cooled(iii)
Matter expands when heated(iv)
b and c are trueQ-9 The conversion of matter from solid to gaseous state is called:
(i)
Condensation(ii)
Sublimation(iii)
Deposition(iv)
EvaporationQ-10 Which of these are amorphous solids?
(i)
Talcum powder(ii)
Plastic(iii)
Flour dough(iv)
All of theseQ-11 Which is true with regard to the odour of ammonia gas?
(i)
Odourless(ii)
Fragrant smell(iii)
Pungent odour(iv)
None of theseQ-12 If a solid can be drawn into wires, it is said to be:
(i)
Malleable(ii)
Ductile(iii)
Elastic(iv)
None of theseQ-13 Which of these will not allow heat or electricity to pass through them?
(i)
Wood(ii)
Plastic(iii)
Rubber(iv)
All of theseQ-14 In water, the molecules pull together into a spherical shape due to the force of:
(i)
Gravity(ii)
Cohesion(iii)
Adhesion(iv)
MagnetismQ-15 The meniscus of mercury is:
(i)
Concave(ii)
Convex(iii)
Horizontal(iv)
None of theseChapter-3 Elements, Compounds and Mixtures
Q-1 Define:
Q-2 Name:
Q-3 Answer the following questions.
Q-4 Classify the following as metals, nonmetals and metalloids
Q-5 Classify the following as elements, compounds and mixtures.
Q-6 Give two examples of each:
Q-7 State whether the following statements are true or false:
Multiple Choice Questions
Q-1 Which of the following is a liquid metal?
(i)
Bromine(ii)
Mercury(iii)
Iodine(iv)
NeonQ-2 There are ___________ gaseous elements known to us.
(i)
9(ii)
10(iii)
11(iv)
12Q-3 There are _________ liquid elements known to us.
(i)
6(ii)
3(iii)
4(iv)
5Q-4 Which of the following is NOT a metalloid?
(i)
Arsenic(ii)
Boron(iii)
Carbon(iv)
SiliconQ-5 Which of the following is NOT a characteristic of non-metals?
(i)
High melting point and boiling point(ii)
Low melting point and boiling point(iii)
Poor conductors of heat and electricity(iv)
Non-malleable and non-ductileQ-6 Which of the following statement is False for metals?
(i)
They have low densities(ii)
They have high densities(iii)
Generally solids at room temperature(iv)
Malleable and ductileQ-7 Silicon becomes a good conductor of electricity when is added to it :
(i)
Boron(ii)
Mercury(iii)
Carbon(iv)
SulphurQ-8 __________ is a form of carbon that conducts electricity:
(i)
Coal(ii)
Graphite(iii)
Diamond(iv)
None of theseQ-9 A water molecule contains hydrogen and oxygen in the ratio:
(i)
1:2(ii)
1: 1(iii)
2:1(iv)
2:2Q-10 When iron is heated with sulphur, the compound formed is:
(i)
Iron sulphite(ii)
Iron sulphide(iii)
Iron sulphate(iv)
None of theseQ-11 The colour of sulphur is:
(i)
Brown(ii)
Red(iii)
Yellow(iv)
PinkQ-12 Hydrogen burns with a __________ flame:
(i)
Yellow(ii)
Blue(iii)
Orange(iv)
GreenQ-13 Which of the following is a characteristic of water?
(i)
It is non combustible(ii)
It is a non supporter of combustion(iii)
It boils at 100°C and freezes at 0°C(iv)
All of theseQ-14 The nitrogen molecule is made up of nitrogen atoms:
(i)
1(ii)
2(iii)
3(iv)
4Q-15 The symbol of sodium is:
(i)
S(ii)
So(iii)
Na(iv)
None of theseChapter-4 Separation of Mixtures
Q-1 Answer the following questions.
Q-2 Some common daily methods of separation are listed below. Select an appropriate method used from the list given. Filtration, Straining, Sieving, Handpicking, Winnowing, Sifting, Washing, Boiling
Q-3 Explain the following terms:
Q-4 Complete the following with respect to separation techniques.
Multiple Choice Questions
Q-1 The method used to separate tea leaves from tea :
(i)
Evaporation(ii)
Filtration(iii)
Straining(iv)
SievingQ-2 The method used to separate pebbles from sand:
(i)
Hand picking(ii)
Sedimentation(iii)
Decantation(iv)
WashingQ-3 The process of transferring the supernatent liquid without disturbing the sediment is called:
(i)
Sedimentation(ii)
Decantation(iii)
Filtration(iv)
SublimationQ-4 A porous medium which allows only the liquid to pass through it is called:
(i)
Sieve(ii)
Strainer(iii)
Filter paper(iv)
All of theseQ-5 The method used to separate salt from sea water:
(i)
Filtration(ii)
Evaporation(iii)
Sublimation(iv)
SedimentationQ-6 Which of the following statements refer to the importance of pure substances?
(i)
Impurities in medicines could be harmful to health(ii)
Impurities in drinking water could cause diseases like typhoid and cholera(iii)
In research work, pure substances give accurate results(iv)
All of the aboveQ-7 Magnetic separation can be carried out if one of the components of the mixture is:
(i)
Sulphur(ii)
Iron(iii)
Salt(iv)
Ammonium chlorideQ-8 For which mixture can sublimation be used to separate the components?
(i)
Iodine + Ammonium chloride(ii)
Powdered camphor + Iodine(iii)
Sodium chloride + Ammonium chloride(iv)
All of theseQ-9 Which of the following will sublime at room temperature?
(i)
Camphor(ii)
Naphthalene(iii)
Iodine(iv)
None of theseQ-10 Filtration is used to separate a mixture of water and:
(i)
Fine insoluble solid(ii)
Fine soluble solid(iii)
Coarse insoluble solid(iv)
None of theseChapter-5 Water
Q-1 Answer the following questions.
Q-2 Give the following physical constants of water:
Q-3 State one characteristic of the following:
Q-4 Explain briefly:
Q-5 Name
Q-6 Select the appropriate letters from the list provided below and label the given diagram.
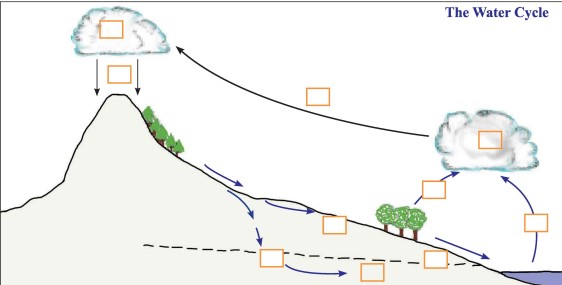
Multiple Choice Questions
Q-1 Water exists in the following state:
(i)
Solid(ii)
Liquid(iii)
Gas(iv)
All of theseQ-2 About ____________ % of our body weight is water.
(i)
65-75(ii)
25-35(iii)
85-95(iv)
15-25Q-3 Which of the following is NOT surface water?
(i)
Spring(ii)
Pond(iii)
River(iv)
OceanQ-4 This is the purest form of natural water:
(i)
River water(ii)
Rainwater(iii)
Seawater(iv)
Well waterQ-5 The salty taste of seawater is due to dissolved:
(i)
Common salt(ii)
Oxygen(iii)
Iron sulphide(iv)
Carbon dioxideQ-6 This is an underground water resource:
(i)
Stream(ii)
Spring(iii)
Pond(iv)
None of theseQ-7 Water which is fit for drinking is called:
(i)
Portable water(ii)
Potable water(iii)
Filtered water(iv)
Mineral waterQ-8 Oceans cover about ________________ % of the Earth's surface.
(i)
30(ii)
15(iii)
50(iv)
70Q-9 Which of the following is a device to raise underground water?
(i)
Hand pump(ii)
Pulley system(iii)
Elevator(iv)
Both (a) and (b)Q-10 Mineral water is sourced from:
(i)
Rivers(ii)
Lakes(iii)
Streams(iv)
SpringsQ-11 Which of the following is a stage in the purification of natural water for drinking?
(i)
Coagulation(ii)
Filtration(iii)
Chlorination(iv)
All of theseQ-12 Which is a water borne disease?
(i)
Typhoid(ii)
Cholera(iii)
Dysentery(iv)
All of theseQ-13 Which of the following is a characteristic of drinking water?
(i)
It is colourless and odourless(ii)
Contains mineral salts(iii)
Contains dissolved 0 2 and CO2(iv)
All of theseQ-14 Which statement is NOT true for water?
(i)
Pure water boils at 100°C and freezes at 0°C(ii)
Pure water is a good conductor of electricity(iii)
The density of pure water is 1 g/cm3(iv)
Water is a universal solventQ-15 Seawater is unfit for drinking because it contains:
(i)
Excess salts(ii)
Excess CO2(iii)
Excess bacteria(iv)
None of theseChapter-6 Air and Atmosphere
Q-1 Answer the following questions.
Q-2 Match the items in column A with items in column B. (More than one matching is possible.)

Q-3 Name a gas that fits the following description
Q-4 State whether the following statements are true or false:
Q-5 Draw neatly labelled sketches to show the following:
Q-6 Answer the following questions.
Q-7 Match the items in column A with items in column B. (More than one matching is possible.)

Q-8 Name a gas that fits the following description
Q-9 State whether the following statements are true or false:
Q-10 Draw neatly labelled sketches to show the following:
Multiple Choice Questions
Q-1 Which of these gases is not present in the Earth's atmosphere?
(i)
Hydrogen(ii)
Ozone(iii)
Argon(iv)
NitrogenQ-2 The ozone layer protects us from:
(i)
Infrared rays(ii)
Ultraviolet rays(iii)
Visible rays(iv)
None of theseQ-3 Nature maintains the proportion of gases in the air through:
(i)
Nitrogen cycle(ii)
Carbon cycle(iii)
water cycle(iv)
All of theseQ-4 Which of the following statement is true about air?
(i)
Air is a mixture of gases(ii)
Air exerts pressure(iii)
Air has mass and it occupies space(iv)
All of theseQ-5 The percentage of nitrogen in the air is about:
(i)
80%(ii)
20%(iii)
60%(iv)
None of theseQ-6 Nitrogen is used by plant to make:
(i)
Carbohydrates(ii)
Proteins(iii)
Fats(iv)
Only (a) and (b)Q-7 Which is a test to identify carbon dioxide gas?
(i)
It relights a glowing splinter(ii)
It extinguishes a glowing splinter(iii)
It turns lime water milky(iv)
Both (b) and (c)Q-8 Which statement is true for oxygen?
(i)
It is 1.5 times heavier than air(ii)
It is 1.5 times lighter than air(iii)
It allows substances to burn in it(iv)
It is highly soluble in waterQ-9 Humidity is the amount of _______________ present in the air.
(i)
Nitrogen(ii)
Oxygen(iii)
Water vapour(iv)
Inert gasesQ-10 Burning of fossil fuels releases ______________ into the atmosphere.
(i)
Nitrogen dioxide(ii)
Sulphur dioxide(iii)
Carbon dioxide(iv)
NitrogenQ-11 Which statement refers to the dangers of global warming?
(i)
Cause glaciers and snow to melt(ii)
Cause floods and climate change(iii)
Cause animals to lose their homes(iv)
All of theseQ-12 During photosynthesis, plant take in the following gas:
(i)
Carbon dioxide(ii)
Oxygen(iii)
Nitrogen(iv)
Both (a) and (c)Q-13 By the process of photosynthesis, green plants prepare food in the form of:
(i)
Carbohydrates(ii)
Proteins(iii)
Fats(iv)
All of theseQ-14 Which is a greenhouse gas?
(i)
Carbon dioxide(ii)
Methane(iii)
Nitrous oxide(iv)
All of theseQ-15 During photosynthesis, plant give out the following gas:
(i)
Carbon dioxide(ii)
Oxygen(iii)
Nitrogen(iv)
Both (a) and (c)