Question bank
Chapter-1 Periodic Properties and Variations of Properties
Q-1
Answer these Questions.
(i) What is the significance of atomic number in the classification of elements?
(ii) Give the names and symbols of the third period elements of the Long Form Periodic Table.
(iii) The atomic radii of three elements P, Q and R of a period of the Periodic Table are 157 pm (picometre), 104 pm and 125 pm respectively. Giving a reason, arrange these elements in the increasing order of atomic numbers in the period.
(iv) Write the electronic structures of atoms of (i) Potassium (ii) Lithium (iii) Fluorine (iv) Chlorine
Use these electronic structures to explain why potassium is more reactive than lithium, and fluorine more reactive than chlorine.
(v) Name the elements of the third period
(vi) Which element in period 3 is likely to react most violently with chlorine?
(vii) Of the eight elements in period 3, which is likely to form a compound of the formula XCl 3 with chlorine?
(viii) Selenium (Se) occurs below sulphur in the Periodic Table. What is the probable formula of the hydrogen compound of selenium?
Q-2
Arrange the following elements in increasing order of their atomic size and ionisation energy:
chlorine, iodine, fluorine, bromine
Q-3
How does the number of valence electrons vary on moving from left to right:
(i) In the first period of the Periodic Table?
(ii) In the second period of the Periodic Table?
Q-4
How do the following properties change on going from left to right in a period of the Periodic Table?
Give examples in support of your answer.
(i) Nature of oxides of the elements.
(ii) Chemical reactivity of the elements.
Q-5
Given below are some of the elements with their relative atomic sizes:

(i) Arrange these elements in the order of increasing atomic numbers (keeping the element with least atomic number first).
(ii) From this data, infer how the atomic size of the elements varies from left to right in a period of the Periodic Table.
Q-6
For the main groups of the periodic table, the metallic properties of the elements vary approximately with their positions as shown in the chart below:
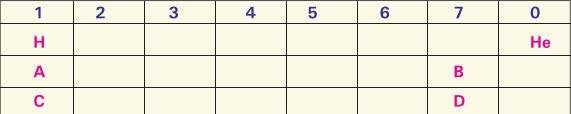
(i) Where will you find the most metallic element?
(ii) Where will you find the most non-metallic element?
(iii) Where will you find the smallest atom?
Q-7
The atomic number of nitrogen is 7 and that of phosphorus is 15.
(i) Write the electronic configuration of nitrogen and phosphorus atoms.
(ii) In which group or groups do these elements occur in the Periodic Table?
Q-8
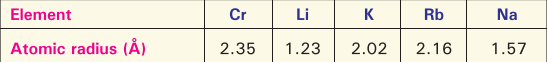
Below are given some elements with their atomic radii:
Infer to which group of the Periodic Table they belong and how the atomic radius varies from top to bottom in a group of the Periodic table.
Q-9
Fill in the blanks by selecting the correct alternative:
(i) A negative ion is (larger/smaller) _________ than its parent atom.
(ii) A positive ion is (larger/smaller)_________ than its parent atom.
(iii) Down a group, the first ionisation energy generally __________ (increases/decreases).
(iv) The majority of elements in the Periodic Table are________ (metals/non-metals)
Q-10
Answer the following questions with reference to group VII A elements (or halogens).
(i) Name and arrange the elements in order of increasing electronegativity.
(ii) Name the radioactive element in this group.
(iii) Which is the most powerful oxidising agent?
(iv) Name four halogen acids.
(v) State which is the strongest acid mentioned in (iv).
Q-11
State whether the following statements are true or false.
(i) Non-metallic character decreases from fluorine to iodine.
(ii) Halogens are good conductors of heat and electricity.
(iii) Lack of iodine in our diet leads to improper functioning of the thyroid gland.
(iv) Bromine is a yellow liquid at room temperature.
(v) The reactivity of halogens decrease from fluorine to iodine.
Q-12
In the Periodic Table given below, lithium, carbon, oxygen and neon are placed in their correct positions. The positions of nine other elements are represented by letters. These letters are not the symbols for the elements concerned.
By reference to the table, answer the following questions:
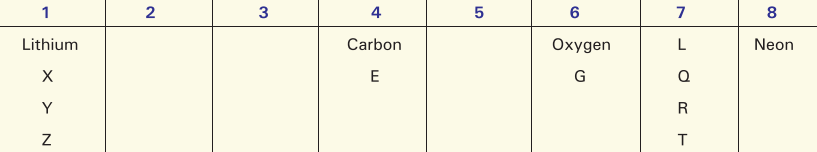
(i) Give the letter of the most reactive metal.
(ii) Give the letter of the most reactive non-metal.
(iii) Name the family of elements represented by L, Q, R, T.
(iv) Name one element in each case occurring in groups 2, 3 and 5.
Q-13
Select the correct alternative:
(i) The atomic radius of sodium atom is (greater/less) than that of a sodium ion.
(ii) Ionisation energy is the amount of energy (absorbed/released) to remove one or more electrons from the valency shell of an isolated gaseous atom.
(iii) The ionisation potential (decreases/increases) going down the group.
(iv) Electron affinity is the amount of energy (absorbed/released) when one or more electron is added to the outermost shell of an isolated gaseous atom.
(v) Smaller the atom, (greater/smaller) is the electron affinity.
(vi) The atomic radius of chlorine atom is (greater/less) than that of a chloride ion.
(vii) (Lithium/Francium) is the lightest element of group IA.
(viii) Elements of the second period which connect their own group diagonally with the next group of the periodic table are called (bridge/transition) elements.
(ix) The fifth period is a long period consisting of (18/32) elements.
(x) The Actinide series is contained in the (sixth/seventh) period.
Q-14
Explain why:
(i) Group IA elements are called Alkali metals.
(ii) Down the group, the atomic radii increases.
(iii) Across a period, the atomic radii decreases.
(iv) Sodium is a metal while sulphur is a non-metal.
(v) Sodium chloride is an ionic compound while carbon tetrachloride is a covalent compound. [Electronegativity values: Na = 0.9; C = 2.5; Cl= 3.0)
Q-15
The following table represents the first three periods of the modern periodic table. Study the table and answer the questions that follow:
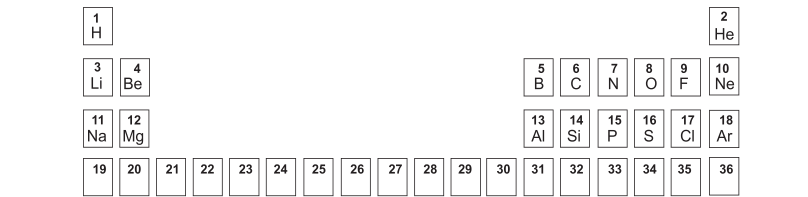
(i) Write the formula of the sulphate of the element with atomic number 13.
(ii) What type of bonding will be present in the oxide of the element with atomic number 1?
(iii) Which feature of the atomic structure accounts for the similarities in the chemical properties of the elements in group 7 A of the periodic table?
(iv) Name the element which has the highest ionisation potential.
(v) How many electrons are present in the valency shell of the element with the atomic number 18?
(vi) What is the name given to the energy released when an atom in its isolated gaseous state accepts an electron to form an anion?
(vii) What is the electronic configuration of the element in the third period which gains one electron to change into an anion?
(viii) Fill in the blanks: The atomic size _______ as we move left to right across the period, because the_________ increases but the __________ remains the same.
Q-16
- In this table H does not represent hydrogen.
- Some elements are given in their own symbol and position in the periodic table.
- While others are shown with a letter.
With reference to the table answer the following questions:
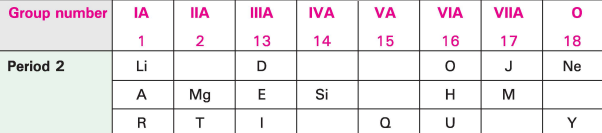
(i) Identify the most electronegative element.
(ii) Identify the most reactive element of group 1 .
(iii) Identify the element from period 3 with least atomic size.
(iv) How many valence electrons are present in Q?
(v) Which element from group 2 would have the least ionisation energy?
(vi) Identify the noble gas of the fourth period .
(vii) In the compound between A and H what type of bond would be formed and give the molecular formula for the same.
Q-17
Study the table below and answer the questions:
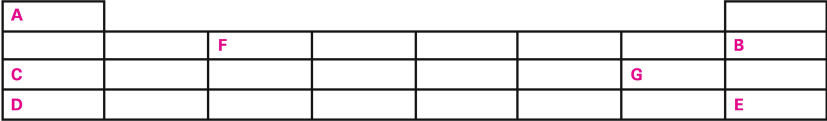
(i) Does A share the same characteristics with the other elements in the same group? Give reasons for your answer.
(ii) Where would you place A ideally?
(iii) Name element D.
(iv) It will combine with only one other element in the table given above. Name the other element and write the formula of the compound between that element and D.
(v) What is the special characteristic of B and E.
(vi) What is the valency of F?
Q-18
Name or State:
(i) Number of Groups in Moseley's Periodic Table
(ii) The period with the highest number of elements
(iii) Period composed of one normal and one inert element
(iv) Group comprising of Inner transition metals
(v) Typical elements are present in the group/period
(vi) The element with atomic number 15 is expected to be in group
(vii) Transition elements are present in the group
(viii) The first element of the Actinide Series
(ix) The most metallic element in group IA
(x) Period/s having 8 elements
Chapter-2 Chemical Bonding
Q-1
Answer these Questions.
(i) What is a chemical bond?
(ii) State the two laws that determine the stability of an atom.
(iii) List three differences between atoms and ions.
(iv) What is an electrovalent or ionic bond?
(v) Which of the following compounds are ionic: NaCl, CaO, H2S, NaH, CH4?
(vi) Show with the help of neat dot and cross diagrams how the ionic compounds named in (v) are formed
(vii) What is a covalent bond? Taking hydrogen chloride and methane as examples, distinguish between a polar covalent bond and a non-polar covalent bond .
Q-2
Explain the following with one example each:
(i) A double covalent bond
(ii) A triple covalent bond
Q-3
Given below is a list of substances:
Ammonia, Aluminium, Nitrogen, Argon, Oxygen, Calcium bromide
Which of the above substances:
(i) Conducts electricity in solid state?
(ii) Is soluble in water?
(iii) Consists of ions.
(iv) Conducts electricity when dissolved in water?
(v) Contains a double bond?
(vi) Contains a triple bond?
(vii) Is monoatomic?
Q-4
Answer the following Questions.
(i) Why do certain compounds form polar covalent bonds? Give three examples of polar covalent compounds.
(ii) Which type of elements form ionic compounds?
(iii) a. What kind of bond is expected between carbon and chlorine?
b. Give an example of a compound formed by these two elements.
c. Write a property of the compound named in (b).
(iv) The atomic number of sodium is 11. What is the number of electrons in Na+?
(v) The atomic number of chlorine is 17. What is the number of electrons in Cl-?
(vi) Does the atomic number of an element change when its atoms form ions? Give reasons for your answer.
(vii) a. How many covalent bonds does an ammonia molecule have? b. Why is it incorrect to say molecules of sodium chloride, but correct to say molecules of ammonia?
(viii) Give one example each of diatomic and triatomic molecules.
(ix) State the meaning of octet configuration.
(x) A metal X (atomic number 19) burns in chlorine to produce a white solid chloride Y. By means of diagrams, illustrate the arrangement of electrons in X, both, before and after the reaction.
Q-5
What are the bond types present in each of the following substances?
(i) Water
(ii) Nitrogen
(iii) Magnesium chloride
(iv) Calcium chloride
Q-6
The elements W, X, Y, and Z have atomic numbers 7, 8, 9, and 11 respectively. Write the formula of the compounds you would expect to form between the following pairs of elements and indicate the type of bonding present:
(i) Wand X
(ii) X and X
(iii) W and Z
(iv) Y and Y
Q-7
For three elements X, Y and Z, the following data are given:
Write the chemical formula and nature of compounds (electrovalent / covalent) formed between:

(i) X and X
(ii) X and Y
(iii) Z and X
Q-8
An element A has four electrons in the outermost shell of its atom and combines with another element B having seven electrons in the outermost shell of its atom. The compound formed does not conduct electricity.
(i) What is the nature of the chemical bond in the compound?
(ii) Give the electron dot structure of its molecule.
Q-9
Answer the following Questions.
(i) State one test by which sodium chloride can be distinguished from carbon tetrachloride.
(ii) An element A has two electrons in the outermost shell of its atom and combines with an element B having seven electrons in the outermost shell forming the compound AB 2 . The compound when dissolved in water conducts electric current. Giving reasons, state the nature of chemical bond in the compound.
(iii) An element A has four valence electrons in its atom whereas element B has one valence electron in its atom. The compound formed by A and B does not conduct electricity. What is the nature of chemical bond in the compound formed? Give its electron dot structure.
(iv) Draw the structural diagram to represent the molecule of each of the following: (a) Hydrogen (b) Oxygen
(v) Mention two differences between hydrogen and oxygen evident from their structural diagrams (atomic number or number of electrons need not be mentioned as a point of difference).
(vi) Both, hydrogen and oxygen are examples of non-polar covalent bond. Explain.
(vii) When hydrogen and oxygen combine, the product water is a polar covalent compound. Explain.
(viii) What is a lone pair of electrons? Draw an electron dot diagram of a hydronium ion and label the lone pair of electron. Name a neutral covalent molecule which contains one lone pair of electrons.
(ix) Name the charged particles which attract one another to form electrovalent compounds.
(x) In the formation of electrovalent compounds, electrons are transferred from one element to another. How are electrons involved in the formation of a covalent compound?
(xi) The electronic configuration of nitrogen is 2, 5. How many electrons in the outer shell of a nitrogen atom are not involved in the formation of a nitrogen molecule?
(xii) In the formation of magnesium chloride (by direct combination between magnesium and chlorine), name the substance that is oxidized and the substance that is reduced.
(xiii) By drawing an electron dot diagram, show the lone pair effect leading to the formation of ammonium ion from ammonia gas and hydrogen ion.
(xiv) There are three elements E, F and G with atomic numbers 19, 8 and 17 respectively. Give the molecular formula of the compound formed between E and G and state the type of chemical bond in this compound.
(xv) Compare the compounds carbon tetrachloride and sodium chloride with regard to solubility in water and electrical conductivity.
(xvi) Why do covalent compounds exist as gases, liquids or soft solids?
(xvii) An element Z has atomic number 16. State the formula between Z and hydrogen.
What kind of a compound is this?
Q-10
A compound is formed between the atoms X and Y. If the atomic number of X is 19 and Y is 8 . Answer the following questions:
(i) State the valency of X and Y respectively.
(ii) What are the ions formed by X and Y called respectively?
(iii) Why do X and Y form ions?
(iv) In a reaction between X and Y explain which atom gets oxidised.
(v) Give the formula and electron dot diagram of the compound formed (if possible) by the following atoms:
a. X and X
b. X and Y
c. Y and Y
Also state the type of bonding formed in each compound.
(vi) Give the formula of the compound formed in (v) that will have/is likely to be
a. A high melting point and boiling point.
b. A true chemical bonding.
c. A gaseous compound.
d. A good conductor of electricity in aqueous or molten state.
e. A high speed of chemical reaction.
Q-11
Give one term/phrase for the following:
(i) Positively charged particles formed from atoms.
(ii) Certain elements which exhibit properties intermediate to those between metals and non-metals.
(iii) Number of electrons present in the outermost shell of an atom.
(iv) Atom with stable electronic configuration.
(v) Negatively charged particle formed from atoms.
(vi) Positively charged particles in the nucleus of an atom.
(vii) Negatively charged particles present in atoms.
Q-12
Match the column A with column B

Chapter-3 Acids, Bases and Salts
Q-1
Define or explain the following terms:
(i) An acid
(ii) pH scale
(iii) An indicator
(iv) Salt
(v) Normal Salt
(vi) Acid salt
Q-2
Give the name and formula of two:
(i) Strong, monobasic acids
(ii) Weak, dibasic acids
(iii) Non-volatile acids
(iv) Volatile acids
Q-3
Give balanced equations to prepare each of the following salts by a different method. Also state the method used.
(i) Anhydrous ferric chloride
(ii) Ferrous chloride
(iii) Sodium chloride
(iv) Lead chloride
(v) Potassium chloride
Q-4
Explain why:
(i) Ferric chloride is stored in air-tight bottles.
(ii) On exposure to air, Glauber's salt loses weight while quicklime gains weight.
(iii) Common salt (containing traces of magnesium chloride) becomes sticky during the monsoons.
(iv) Blue copper sulphate crystals turn white on heating.
(v) Anhydrous calcium chloride is used in desiccators.
(vi) Cone. sulphuric acid becomes dilute when exposed to air.
(vii) Universal indicator is preferred to acid-base indicators.
Q-5
What would you observe when:
(i) Red litmus paper is introduced into a solution of sodium sulphate.
(ii) Methyl orange is added to dilute hydrochloric acid.
(iii) Iron nails are kept in potassium chloride solution.
(iv) A drop of phenolphthalein is added to a solution of lime water.
(v) Blue litmus is introduced into a solution of ferric chloride.
Q-6
Answer the following Questions.
(i) Acetic acid is a monobasic acid. Explain.
(ii) State three applications of the pH scale.
(iii) Using lead(II) nitrate solution, how would you confirm whether a given colourless solution is hydrochloric acid, sulphuric acid or nitric acid?
(iv) Arrange the following in order of increasing acidity:
Seawater; Blood; Milk; Hydrochloric acid; Lime juice; Acid rain
(v) Sulphuric acid is said to be a dibasic acid. Explain the term 'dibasic'?
(vi) What do you understand by the statement 'Acetic acid, CH3COOH, is a monobasic acid'?
(vii) Two acids A and B have pH values of 1 and 5 respectively. Which is the stronger acid, A or B?
(viii) The pH value of pure water is 7. Compare the pH value of sulphur dioxide solution and ammonia solution with that of pure water.
Q-7
Name:
(i) A gas that dissolves in water to yield an alkaline solution.
(ii) A white solid that dissolves in water to yield an alkaline solution.
(iii) An acid that occurs in fats.
(iv) A tribasic acid.
(v) A hydrated hydrogen ion.
Q-8
Answer the following questions relating to salts and their preparation:
(i) What is a 'salt'?
(ii) What kind of salt is prepared by precipitation? (Double decomposition)
(iii) Name a salt prepared by direct combination. Write the equation for the reaction that takes place when preparing the salt you have named.
(iv) What procedure would be used to prepare a sodium salt such as sodium sulphate? (Give the name of the procedure only)
Q-9
Prepare the salts A, B, C and D by a suitable method which relates to the description given below:
(Do not describe the procedure for the preparation. Refer only to the appropriate method)
(i) A is a sodium salt.
(ii) B is an insoluble salt.
(iii) C is a soluble salt of copper.
(iv) D is a soluble salt of zinc.
Q-10
Name:
(i) Two bases which are not alkalis.
(ii) A normal salt and an acid salt of the same acid.
(iii) A salt insoluble in cold water but soluble in hot water.
Q-11
Name from the list of substances given below, the substances which you would use to prepare each of the following salts named in parts (i) to (iv). The substances are:
Copper, lead, sodium, zinc, copper oxide, lead carbonate, sodium carbonate solution, dilute hydrochloric acid, dilute nitric acid and Dilute sulphuric acid:
(i) Zinc sulphate
(ii) Copper sulphate
(iii) Sodium sulphate
(iv) Lead sulphate
Q-12
A is a soluble acidic oxide; B is a soluble base. Compared to the pH of pure water, what will be the pH of:
(i) a solution of A?
(ii) a solution of B?
Q-13
Answer the questions below, relating your answers only to salts in the following list:
Sodium chloride, anhydrous calcium chloride, copper sulphate-5-water.
(i) What name is given to the water in the compound copper sulphate-5-water?
(ii) If copper sulphate-5-water is heated, the water is driven off leaving anhydrous copper sulphate.
a. What is the colour of anhydrous copper sulphate?
b. By what means, other than heating, could you dehydrate copper sulphate-5-water and obtain anhydrous copper sulphate?
(iii) Which one of the salts in the given list is deliquescent?
Q-14
From the list of substances given below, choose the pair required to prepare the salts (1) to (3) in the laboratory and write down the relevant equations. The substances are:
Chlorine, iron, lead, lead nitrate solution, sodium nitrate solution, iron(III) carbonate, lead carbonate, iron(III) hydroxide, sodium hydroxide solution and dilute hydrochloric acid
The salts are:
(i) Sodium chloride
(ii) Lead chloride
(iii) Anhydrous iron(III) chloride
Q-15
Answer the following questions, relating your answers only to salts in the list given below:
Anhydrous calcium chloride, copper sulphate-5-water, sodium carbonate-10-water
(i) Which compound is efflorescent?
(ii) Which compound is blue in colour?
(iii) Which compound is deliquescent?
(iv) What would be seen on mixing a solution of calcium chloride with a solution of sodium carbonate?
(v) Write the balanced equation for the reaction occurring when a solution of calcium chloride is mixed with solution of sodium carbonate.
Q-16
Give the equation for the preparation of each of the following salts from the starting material given.
(i) Copper sulphate from copper(II) oxide.
(ii) lron(III) chloride from Iron.
(iii) Potassium sulphate from potassium hydroxide solution.
(iv) Lead chloride from lead carbonate (two equations).
Q-17
Write the balanced chemical equation for each of the following reactions:
(i) Zinc sulphide is reacted with dilute hydrochloric acid.
(ii) Calcium bicarbonate reacts with dilute hydrochloric acid.
(iii) Dilute sulphuric acid is poured over sodium sulphite.
(iv) Lead nitrate solution is added to sodium chloride solution.
(v) Zinc is heated with sodium hydroxide solution.
Q-18
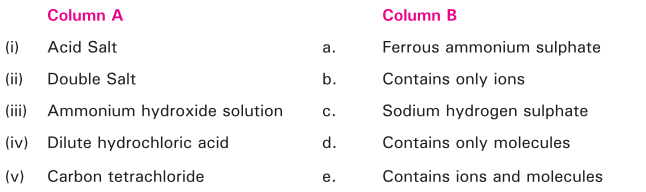
Match the following:
Q-19
Give one example of each:
(i) A deliquescent salt
(ii) A hygroscopic liquid
(iii) An efflorescent salt
(iv) A solid drying agent
(v) An acid salt
(vi) A tribasic acid
Chapter-4 Analytical Chemistry
Q-1
Answer the following Questions.
(i) Sodium hydroxide solution is added to solution (A), a white precipitate is formed which is insoluble in excess sodium hydroxide solution. What is the metal ion present in solution (A)?
(ii) When ammonium hydroxide is added to solution [B], a pale blue precipitate is formed. This pale blue precipitate dissolves in excess ammonium hydroxide giving an inky blue solution. What is the cation present in solution [B]? What is the probable colour of solution [B]?
(iii) Explain your observations when ammonium hydroxide is add slowly, until in excess, to a solution of calcium nitrate.
Q-2
Write the observations for the following reactions:
(i) Sodium hydroxide is added drop wise till in excess to a solution of : a. Zinc sulphate
b. Lead nitrate
c. Calcium chloride
(ii) Ammonium hydroxide is added first in a small quantity and then in excess to a solution of copper sulphate.
Q-3
You are given the three white powders calcium carbonate, lead carbonate and zinc carbonate.
(i) Describe the tests you would carry out in solution to identify the metal in each of the above compounds.
(ii) Indicate clearly how you would prepare the solutions for the tests.
Q-4
Write the equation for each of the following reactions:
(i) Solutions of ammonium chloride and sodium hydroxide are mixed and heated.
(ii) Copper sulphate solution is added to sodium hydroxide solution.
Q-5
Write balanced equations for the following reactions:
(i) lron(III) chloride solution with sodium hydroxide solution.
(ii) Zinc chloride and excess ammonium hydroxide solution.
(iii) Ammonium hydroxide is added in excess to a solution of copper sulphate.
(iv) Sodium hydroxide is added in excess to a solution of zinc sulphate.
(v) Sodium hydroxide is added in excess to a solution of lead nitrate.
Q-6
Sodium hydroxide solution is added to the solutions containing the ions mentioned in List X. List Y gives the details of the precipitate. Match the ions with their coloured precipitates.

Chapter-5 Mole Concept and Stoichiometry
Q-1
Answer the following Questions based on Gay- Lussac's Law
(i) State: Gay-Lussac's Law of combining volumes.
(ii) One litre of oxygen at STP is made to react with three times the volume of carbon monoxide at STP. Find the composition of the resultant mixture.
(iii) 30 mL of methane is mixed with 80 mL of oxygen and burnt. If all measurements are made at room temperature and constant pressure, what is the volume of the unreacted oxygen?
(iv) Assuming that air contains one-fifth by volume of oxygen, find the volume of gaseous mixture left after the explosion of a mixture of 1 litre of carbon monoxide and 10 litre of air.
(v) Find the volume of oxygen at STP required for complete combustion of 2 litres of carbon monoxide at STP.
(vi) 60 cm3 of oxygen was added to 24 cm3 of carbon monoxide and the mixture ignited. Calculate:
(a) the volume of oxygen used up.
(b) the volume of carbon dioxide formed.
Q-2
In the preparation of ammonia in industry, the raw materials are nitrogen and hydrogen. These are mixed together in the correct proportions needed to form ammonia.
(i) How much hydrogen would there be in 400 litres of the gaseous mixture required for the manufacture of ammonia?
(ii) If all the N2 and H2 used were converted into ammonia, find the volume of the ammonia formed.
(iii) How would the volume of ammonia formed compare with the volume of the original mixture?
Q-3
Answer the folowing Quetions based on Weight- Volume Relationship.
(i) How many litres of hydrogen will be liberated at STP when 2.8 g of iron react with sulphuric acid?
[Fe= 56, H = 1]
(ii) What weight of zinc and sulphuric acid would be required to produce enough hydrogen to completely reduce 8.5 g of copper (II) oxide to copper?
[Zn= 65.3, Cu= 63.5, S = 32, 0 = 16]
(iii) It was found that 380 mL of a gas at 27°C and 800 mm pressure weighed 0.455g. Find the molecular weight of the gas.
(iv) What mass of silver chloride will be obtained by adding an excess of hydrochloric acid to a solution of0.34 g of silver nitrate?
[Ag= 108, N = 14, Cl= 35.5, 0 = 16, H = 1]
(v) What volume of oxygen at STP will be obtained by the action of heat on 20 g of potassium chlorate?
[K = 39, 0 = 16, Cl= 35.5]
Q-4
Answer the folowing Questions Based on Avogadro Law and Mole Concept.
(i) Differentiate between gram-atom and gram-molecule.
(ii) Calculate the number of moles in 0.56 g of iron.
(iii) How many molecules are present in 22 g of CO2?
(iv) Calculate the volume occupied at NTP by 14 g of nitrogen.
(v) Determine the molecular mass of a gas, if 5 g of it occupy a volume of 4 litres at STP
(vi) Compare the number of atoms in 10 g of chlorine with that of 10 g of nitrogen.
Q-5
Fill in the blanks:
(i) Mass of 22.4 litres of gas at STP is ________.
(ii) One gram-atom of an element contains ____ atoms.
(iii) One amu is the mass of _______ atom of C12.
(iv) Avogadro's number is equal to ______.
Q-6
State whether the following statements are true or false.
(i) Unit of molecular weight is gram.
(ii) One mole is the short form of a molecule.
(iii) 1 g-atom of an element means atoms present in 1 g of the element
(iv) Equal volumes of all gases at the same temperature and pressure contain equal number of atoms.
Q-7
How many grams are there in:
(i) 2 moles of molecular oxygen?
(ii) 0.01 mole of molecular nitrogen?
Q-8
How many mole-atoms are there in:
(i) 112 g of iron [Fe =56]
(ii) 48 g ofoxygen [O = 16]
Q-9
What is the volume occupied by the following gases at STP?
(i) 48 g of oxygen.
(ii) 16 g of sulphur dioxide
Q-10
What is the relative molecular mass of a gas, if the mass of 1 litre of it measured at STP is:
(i) 0.0899 g
(ii) 1.96 g
Q-11
Answer the Following Questions based on Empherical Formula and Molecular Formula.
(i) Differentiate between empirical formula and molecular formula.
(ii) An organic compound contains 40 % carbon, 6.67 % hydrogen and the rest oxygen. If its molecular weight is 180, find its molecular formula
[ C = 12, H = 1, 0 = 16].
(iii) An organic compound has the following percentage composition:
C = 12.76%, H=2.13%, Br= 85.11 % .
The vapour density of the compound is 94. Find out its molecular formula. ( C = 12, H = 1, Br= 80 ).
(iv) A compound is 54.5% carbon, 9.1 % hydrogen and 36.4% oxygen. If the molecular mass of the compound is 88, what is its molecular formula?
(v) Find the empirical formula of a compound of carbon and hydrogen which contains 80% carbon. If the molecular weight of the above compound is 30, what is its molecular formula?
(vi) A gas cylinder filled with hydrogen holds 5 g of the gas. The same cylinder holds 85 g of a gas X under the same temperature and pressure. Calculate the vapour density and mol. wt. of the gas X.
(vii) Silicon (Si= 28) forms a compound with chlorine (Cl= 35.5), in which 5.6 g of silicon is combined with 21.3 g of chlorine. Calculate the empirical formula of this compound.
(viii) A compound of carbon, hydrogen and oxygen is found to contain 40% of carbon, 6.7 % of Hydrogen and 53.3% of Oxygen. Calculate its empirical formula. If its VD is 30, find its molecular formula.
(ix) A gas cylinder can hold 1 kg of hydrogen a room temperature and pressure.
(a) What weight of carbon dioxide can it hold under similar conditions of temperature and pressure?
(b) If the number of molecules of hydrogen in the cylinder is X, calculate the number of carbon dioxide molecules in the cylinder. Give reasons for your answer.
(x) A compound contains 87.5% by mass of nitrogen and 12.5% by mass of hydrogen. Determine the empirical formula of this compound.
(xi) An acid of phosphorus has the following percentage composition:
2.47% hydrogen, 38.27% phosphorus, 59.26% oxygen.
Find the empirical formula of this acid and its molecular formula, given that its relative molecular mass is 162. (H=l, 0=16, P=31).
(xii) An experiment showed that in a lead chloride solution, 6.21 g oflead combined with 4.26 g ofchlorine. What is the empirical formula of this chloride? (Pb= 207, Cl= 35.5)
(xiii) A compound is formed by 24 grams ofX and 64 grams of oxygen. IfX = 12 and O = 16, calculate the simplest formula of the compound.
(xiv) A hydrocarbon has the following percentage composition: Hydrogen= 2.2%, Carbon= 26.6% and Oxygen= 71.2%.
Calculate the empirical formula of the compound. If its molecular weight is 90, find its molecular formula.( H = 1, C = 12, 0 = 16 ).
Chapter-6 Electrolysis
Q-1
Name the ions present, and the products formed at the cathode and anode during electrolysis of:
(i) Molten lead bromide
(ii) Copper(II) sulphate (using copper anode)
Q-2
Name a substance which when dissolved in water contains:
(i) Only ions
(ii) Ions and molecules
Q-3
Answer these Questions.
(a) State your observations for the following electrolytic reactions:
(i) Solid copper sulphate is electrolysed between platinum electrodes.
(ii) Aqueous copper sulphate is electrolysed between platinum electrodes.
(iii) Aqueous copper sulphate is electrolysed between copper electrodes.
(b) Explain briefly each of the observations in 7 (a).
(c) Summarize the electrode reactions for the reactions taking place in 7 (a).
Q-4
With respect to the electrolysis of lead bromide, answer the following questions:
(i) Why is the lead bromide maintained in a molten state?
(ii) Why is the electrolytic cell made of silica?
(iii) Name the ions present in the electrolyte.
(iv) State what you would observe at the:
a. cathode
b. anode
(v) Summarize the electrode reactions.
Q-5
With reference to the electrolysis of acidulated water, answer the following:
(i) Explain why distilled water is a non-electrolyte.
(ii) What is the electrolytic cell called?
(iii) State what you would observe at the
a. cathode
b. anode
(iv) Summarize the electrode reactions.
Q-6
With reference to electroplating, answer the following:
(i) Why are articles electroplated?
(ii) During electroplating, explain why:
a. A direct current is used.
b. A small current should be used for a longer time.
c. The article to be electroplated should always be placed at the cathode.
d. The anode must be made of the metal with which the article has to be plated.
e. What ions must be present in the electrolyte?
Q-7
Explain the terms:
(i) Electrorefining
(ii) Electrometallurgy
(iii) Anode mud
Q-8
Answer these Questions.
(i) Name the cathode and the anode used when:
a. Sodium is extracted from molten sodium chloride.
b. Aluminium is extracted from molten aluminium oxide.
(ii) Summarize the electrode reactions for each of the above mentioned extractions.
(iii) Explain why metals above zinc in the activity series of metals are extracted from their ores by electrolysis.
Q-9
Electrolysis is used in the purification of metals. Name:
(i) The anode
(ii) The cathode
Q-10
Answer the following questions about electroplating a copper wire with silver.
(i) What ions must be present in the electrolyte?
(ii) Of what substance must the anode be made?
(iii) What will be made the cathode?
(iv) What is the equation for the reaction which takes place at the cathode?
Q-11
Give one example in each case of a substance which contains:
(i) ions
(ii) molecules only
(iii) both ions and molecules
Q-12
The following questions refer to the electrolysis of copper sulphate solution with copper electrodes:
(i) Compare the change in mass of the cathode with the change in mass of the anode.
(ii) What is seen to happen to the colour of the copper sulphate solution if platinum electrodes are used? Explain this observation.
(iii) What is the practical application of the electrolysis of copper sulphate solution? Briefly describe one such application.
Q-13
Answer these Questions.
(i) What should be the physical state of the lead bromide if it is to conduct electricity?
(ii) What particles are present in pure lead bromide?
(iii) Write the equations for the reactions which take place at the electrodes during the electrolysis of lead bromide.
(iv) As we descend the electrochemical series containing cations, the tendency of the cations to get (oxidised/reduced) ______ at the cathode increases. The (higher/lower) ______ the concentration of an ion in a solution, the greater is the probability of its being discharged at its appropriate electrode.
(v) What kind of particles will be found in a liquid compound which is a non-electrolyte?
(vi) If HX is a weak acid, what particles will be present in its dilute solution apart from those of water?
(vii) What ions must be present in a solution used for electroplating a particular metal?
(viii) Explain how electrolysis is an example of redox reaction.
(ix) Explain why copper, though a good conductor of electricity, is a non-electrolyte.
Q-14
Choosing only words from the following list write down the appropriate words to fill in the blanks (i) to (v) below:
anions, anode, cathode, cations, electrode, electrolyte, nickel, voltameter
To electroplate an article with nickel requires an (i) ______ which must be a solution containing (ii) ______ions. The article to be plated is placed as the (iii) ______ of the cell in which the plating is carried out. The ______ (iv) ______ of the cell is made from pure nickel. The ions which are attracted to the negative electrode and discharged are called (v) _____
Q-15
Explain the terms:
(i) Electrolysis
(ii) A strong electrolyte
(iii) Anode
(iv) Ion
Q-16
Answer the following Questions.
(i) Explain the following statement: Molten sodium chloride is decomposed by an electric current.
(ii) Explain why sodium chloride in the molten or in solution state conducts electricity but in the solid state does not conduct.
(iii) What is the difference between an electrolyte and a non-electrolyte?
(iv) State three applications of electrolysis.
(v) Make a neatly labelled sketch to show how a brass spoon can be plated with silver. Explain your choice of electrolyte used.
Chapter-7 Metallurgy
Q-1
From the metals copper, iron, magnesium, sodium and zinc, select a different metal in each case which:
(i) Does not react with dilute hydrochloric acid.
(ii) Can form 2 + and 3 + ions.
(iii) Has a hydroxide that reacts with both acids and alkalis.
(iv) Does not react with cold water but reacts with steam when heated.
Q-2
Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
(i) Write three balanced equations for the purification of bauxite by Baeyer process.
(ii) Name a chemical used for dissolving aluminium oxide. In which state of subdivision is the chemical used?
(iii) Write an equation for the reaction which takes place at the anode during the extraction of aluminium by the electrolytic process.
Q-3
Match the properties and uses of alloys in List 1 with the appropriate answer from List 2.

Q-4
From the options given alongside each statement, select the most appropriate.
(i) A metal that gets covered with a protective film of oxide when exposed to air
Al, Cu, Mg
(ii) A metal which burns in air with a golden flame
Zn, K, Na
(iii) A metal that can displace hydrogen from boiling water and steam
K, Zn, Fe
(iv) (iv) A metal that does not react with air at room temperature
Na, Mg, Ca
(v) A metal that reacts reversibly with steam
Al, Na, Fe
Q-5
From your knowledge of the activity series, name a metal:
(i) Which reacts readily with cold water giving hydrogen.
(ii) Which displaces hydrogen from dilute sulphuric acid or hydrochloric acid.
(iii) Whose hydroxide is a strong base.
(iv) Whose carbonate does not decompose on heating.
(v) Which displaces iron from iron (Ill) oxide or ferric oxide.
Q-6
Answer the Following Questions.
(i) You are given samples of three metals: sodium, magnesium and copper. Suggest any two activities to arrange them in order of their decreasing reactivity.
(ii) A metal X in the form of turnings reacted with a hot, concentrated dibasic acid H2 Y and produced a deep blue solution of salt XV. When a wire of metal Z is put in the solution of XV, the deep blue colour was slowly discharged, a colourless ZY salt solution was formed and X was generated. Explain the observation.
(iii) The following information concerns three metals: X, Y, and Z.
- Z will displace X from a solution of its nitrate.
- Y is obtained by the electrolysis of its molten chloride.
- Z is obtained by heating its oxide with carbon
Arrange X, Y and Z in the correct order of reactivity, the most reactive being placed first.
(iv) Place the following metals in the order in which they appear in the activity series starting with the most reactive:
lead, sodium, iron, zinc.
(v) Name two metals normally manufactured by the electrolysis of fused compounds.
(vi) Name the two compounds used above.
(vii) For one of the above metals, explain the reaction taking place in the cathode.
(viii) Name the most abundant metal on the Earth's crust.
(ix) Name any two metals that are found in the free or native state. Explain why.
(x) Distinguish between a mineral and an ore.
(xi) Name the four main steps involved in the extraction of a metal from its ore.
(xii) Mention any two characteristics of alloys.
(xiii) Name one important component of brass.
Q-7
The following questions are relevant to the extraction of Aluminium:
(i) State the reason for addition of caustic alkali to bauxite ore during purification of bauxite.
(ii) Give a balanced chemical equation for the above reaction.
(iii) Along with cryolite and alumina, another substance is added to the electrolyte mixture. Name the substance and give one reason for its addition.
Q-8
Thin strips of three different metals A, Band Care known to be magnesium, copper and iron respectively.
(a) Write down what you would observe in each case when the metals are treated as follows:
(i) When each metal is heated in air.
(ii) When each metal is treated with dilute hydrochloric acid and warmed if necessary.
(iii) When each metal is added to an aqueous solution of zinc sulphate.
(b) Arrange the metals A, Band C in the descending order of activity.
Q-9
Answer the following questions:
(i) Name a metal which is found abundantly in the earth's crust.
(ii) What is the difference between calcination and roasting?
(iii) Name the process used for the enrichment of sulphide ore.
(iv) Write the chemical formula of one main ore of iron and aluminium.
(v) Write the constituents of electrolyte for the extraction of aluminium.
Q-10
Name the metals that can be extracted from the following ores. Also write the formula of the ore.
(i) Bauxite
(ii) Zincite
(iii) Corundum
(iv) Haematite
(v) Zinc blende
(vi) Iron pyrites
Q-11
State the principle underlying the following methods of concentrating the ore and name one ore that is concentrated by the said method.
(i) Hydraulic washing
(ii) Froth flotation
(iii) Magnetic separation
Q-12
Give reasons:
(i) Aluminium is more abundant than gold in the Earth's crust, yet it is the gold and not aluminium that has been known to human since ancient times.
(ii) Metal sulphides occur mainly in rocks and metal halides occur mostly in seas and lakes.
(iii) A sulphide ore is converted to its oxide to extract the metal.
Q-13
Answer these Questions.
(i) Name the principal ore of aluminium.
(ii) Explain the stages in the purification of the ore, giving balanced equations.
(iii) Why is cryolite added to the molten electrolyte before electrolysis?
(iv) Summarize the electrode reactions taking place during the electrolysis of molten alumina.
Q-14
Explain why:
(i) Aluminium cannot be extracted by reducing alumina with carbon.
(ii) In the electrolysis of molten alumina, the carbon anode is gradually consumed.
(iii) A layer of powdered coke is sprinkled on the surface of the electrolyte during the electrolysis of molten aluminium.
(iv) Name two important alloys of aluminium. State their composition and one important use of each.
Q-15
State how the following metals affect the properties when alloyed with another metal:
(i) Manganese
(ii) Zinc
(iii) Tin
(iv) Silicon
(v) Magnesium
Q-16
Give the name and formula of:
(i) A carbonate ore of iron
(ii) A sulphide ore of iron
(iii) An oxide ore of iron
Q-17
Name an alloy of:
(i) Aluminium used in aircraft construction.
(ii) Lead used in electrical wiring or electrical work in joining metals.
(iii) Copper in electrical appliances or household vessels.
(iv) Steel of daily use.
Q-18
Calcium, Copper, Lead, Aluminium, Zinc, Chromium, Magnesium, Iron. Choose the major metals from the list given above to make the following alloys:
(i) Stainless steel
(ii) Brass
Chapter-8 Hydrogen Chloride
Q-1
Give a balanced equation to obtain hydrogen chloride starting with:
(a) Chlorine
(b) Common salt
Q-2
Answer the following questions:
(a) In the laboratory preparation and collection of hydrogen chloride, concentrated sulphuric acid is used twice - once before the gas is prepared and once after the gas is prepared. State the role of cone. sulphuric acid in each case.
(b) How is hydrogen chloride gas collected in the laboratory?
(c) Explain the use of the funnel arrangement in the preparation of aqueous hydrogen chloride.
(d) Name the gas evolved when dilute hydrochloric acid is added to:
(i) Zinc metal
(ii) Calcium carbonate
(iii) Sodium sulphite
(iv) Lead (II) sulphide
(v) Lead (II) sulphide
(vi) Potassium bisulphite
(e) Give a balanced equation for each of the above reactions.
(f) What is aqua regia?
(g) State the use of aqua regia.
(h) What is the function of HCI in the preparation of aqua regia?
(i) State the conditions under which the following substances react.
(i) Hydrochloric acid and nitric acid.
(ii) Hydrogen and chlorine
(iii) Hydrochloric acid and a metal oxide to yield chlorine as one of the products.
(j) Give a balanced equation for each of the reaction taking place in 1 (a).
(k) Hydrochloric acid is used in the laboratory preparation of the following gases:
(i) Carbon dioxide
(ii) Sulphur dioxide
(iii) Chlorine
(iv) Hydrogen sulphide
(l) Give a balanced equation to prepare each of the gases named above.
(m) What property of hydrochloric acid is illustrated by these reactions?
(n) (i) What must be added to sodium chloride to obtain hydrogen chloride?
(ii) Write the equation for the reaction which takes place in (n) (i) above.
(iii) What would you see when hydrogen chloride mixes with ammonia?
(o) Hydrogen chloride dissolves in water forming an acidic solution.
(i) Name the experiment which demonstrates that hydrogen chloride is very soluble in water.
(ii) Give three distinct tests (apart from using an indicator) you would carry out w ith this solution to illustrate the typical properties of an acid.
(p) Write the equation for the reaction of hydrochloric acid w ith each of the following :
(i) Lead nitrate solution
(ii) Manganese(IV) oxide
(q) What happens when dilute hydrochloric acid is added to lead nitrate solution?
Q-3
State your observations when:
(a) A rod dipped in ammonium hydroxide is brought near the mouth of a jar containing hydrogen chloride gas.
(b) Hydrochloric acid is added to a solution of silver nitrate.
(c) Cone. hydrochloric acid is added to manganese dioxide
(d) Moist blue litmus paper is introduced into a jar of hydrogen chloride.
(e) Hydrochloric acid is added to copper(II) carbonate.
Q-4
Name/State:
(a) The type of reaction between a base and an acid .
(b) A white compound which is insoluble in nitric acid but soluble in ammonium hydroxide.
(c) The soluble compound formed in (b).
(d) The ions formed when hydrogen chloride dissolves in water.
(e) The type of bond present between hydrogen and chlorine in hydrogen chloride.
(f) The temperature above which HCI decomposes.
Q-5
State whether the following statements are true or false:
(a) Hydrochloric acid is a dibasic acid.
(b) Hydrochloride is lighter than air.
(c) Hydrogen chloride is sparingly soluble in water.
(d) Hydrogen chloride is combustible, but a non-supporter of combustion .
(e) Dry hydrogen chloride gas is neutral to moist litmus.
Q-6
A solution of hydrogen chloride in water is prepared. The following substances are added to separate portions of the solution. Complete the table by writing the gas evolved in each case and its odour.

Q-7
At the start of the experiment, dry hydrogen gas is filled in the flask.
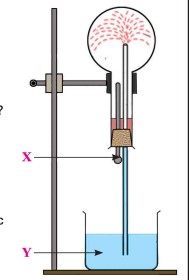
(a) Name the experiment that has been performed.
(b) Name solution Y.
(c) What is the purpose of X?
(d) Which property of hydrogen chloride is demonstrated by this experiment?
(e) If X contained a solution of sodium hydroxide:
a. Would there be any change in the observation?
b. What property of hydrogen chloride would be demonstrated?
(f) What is the type of reaction between sodium hydroxide and hydrochloric acid called?
(g) Name the ions formed when hydrogen chloride dissolves in water.
Chapter-9 Ammonia
Q-1
Answer the following Questions;
(a) State two methods by which ammonia is generally prepared in the laboratory.
(b) Give a balanced equation to illustrate each of the above mentioned methods.
(c) Name the drying agent used for drying the gas. State your reasons for selecting the named drying agent.
(d) How is ammonia gas collected?
(e) Give a chemical test to identify:
a. Ammonia gas
b. Aqueous ammonia
c. Ammonium radical
(f) State two advantages of using the funnel arrangement while preparing aqueous ammonia.
(g) Name the method used for obtaining ammonia on a large scale.
(h) Give a balanced equation for the reaction.
(i) What is the actual ratio of the reactants?
(j) Are the reactants necessarily taken in the same ratio? Explain.
(k) The reaction is reversible and exothermic. Explain the underlined terms.
(l) The temperature used is 450°C. Explain why:
a. A lower temperature is not used.
b. A higher temperature is not used.
(m) What is the pressure applied?
(n) Name the catalyst used. State its function.
(o) What is a promoter? Name the promoter used in this process.
(p) In what form is the ammonia gas stored?
(q) Name the products formed when:
(i) Ammonia is burnt in oxygen.
(ii) A mixture of ammonia and oxygen is passed over heated platinum.
(r) Give a balanced equation for each of the above reactions .
(s) Explain briefly: Dry or liquid ammonia is neutral to litmus.
(t) State your observations when excess ammonium hydroxide is added to:
(i) Ferrous chloride solution
(ii) Ferric chloride solution
(iii) Copper (II) sulphate solution
(iv) Zinc sulphate solution
(v) Lead (II) nitrate solution
(u) Give a balanced equation for each of the above reactions.
(v) State briefly how ammonia is converted to urea. Also give a balanced equation for the reaction.
(t) State your observations when excess ammonium hydroxide is added to:
(i) Ferrous chloride solution
(ii) Ferric chloride solution
(iii) Copper
(II) sulphate solution
(iv) Zinc sulphate solution
(v) Lead (II) nitrate solution
Q-2
Give a balanced equation to show:
(a) The basic nature of ammonia
(b) The reducing property of ammonia
Q-3
List the properties of ammonia that make it:
(a) A good refrigerant
(b) A cleansing agent
Q-4
Give the formula of:
(a) Liquid ammonia
(b) Liquor ammonia
(c) Liquor ammonia fortis
Q-5
Identify substances A, B, C, D and E in the following reactions. Also give a balanced equation for each conversion.
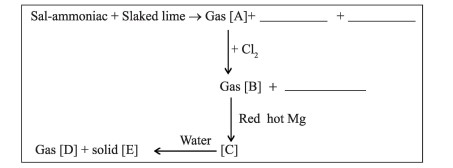
Q-6
The following questions are based on the preparation of ammonia gas in the laboratory:
(a) Explain why ammonium nitrate is not used in the preparation of ammonia.
(b) Name the compound normally used as a drying agent during the process.
(c) How is ammonia gas collected?
(d) Explain why it is not collected over water.
Q-7
Answer the following questions:
(a) Write equations for the following reactions:
(i) Burning of ammonia in oxygen.
(ii) Catalytic oxidation of ammonia.
(b) (i) What would you see in (a) (i) above?
(ii) Name the catalyst used in (a) (ii).
(iii) In the reaction referred to in (a) (ii) the catalyst glows red hot. Why?
(iv) What is the name of the industrial process which starts with the reaction referred to in (a) (ii)?
(c) (i) How soluble is ammonia in water?
(ii) Give two reasons to show that the solution of ammonia in water contains hydroxide ions.
(iii) Name a simple method you would employ to prepare ammonium salts in your laboratory.
(d) All ammonium salts are decomposed on heating. What other property do ammonium salts have in common?
Q-8
Choose the correct word or phrase from the brackets to complete the following sentences:
(a) Ammonium chloride is a soluble salt prepared by (precipitation/neutralisation).
(b) When ammonium chloride is heated, it undergoes (thermal decomposition/thermal dissociation).
(c) Heating ammonium chloride with sodium hydroxide produces (ammonia/nitrogen).
Chapter-10 Nitric Acid
Q-1
Give balanced equations to prepare nitric acid starting with the following (state conditions wherever required).
(a) Nitrogen
(b) Ammonia
(c) Nitre
(d) Lead (II) nitrate
Q-2
Answer the following question
(a) With respect to the laboratory preparation of nitric acid using sodium nitrate and cone. sulphuric acid, explain the following :
(i) The apparatus is made of all glass.
(ii) The reaction mixture is not heated beyond 200°C.
(iii) Pure nitric acid is colourless, but that obtained in the laboratory is slightly yellow.
(b) (i) How is nitric acid collected in the laboratory?
(ii) State two ways the yellow coloration can be removed from the nitric acid.
(c) Name the process by which nitric acid is obtained on a large scale.
(d) Name the reactants used.
(e) State the ratio in which the reactants are taken.
(f) a. Name the catalyst used.
b. Up to what temperature is the catalyst heated?
c. Give the reaction taking place in the catalyst chamber.
d. Name the products formed.
(g) State the function of the cooling tower.
(h) Give a balanced equation for the reaction taking place in:
a. The oxidising chamber
b. The absorption chamber
(i) What is the strength of the nitric acid formed?
(j) How can 98% pure nitric acid be obtained?
(k) Give a chemical test to distinguish between dilute nitric acid and cone. nitric acid.
(l) Name a metal that reacts with very dilute nitric acid to liberate hydrogen. Also give a balanced equation.
Q-3
With respect to the large scale method of obtaining nitric acid, explain the following:
(a) Excess air is taken in the reaction.
(b) The gases entering the catalyst chamber must be pure.
(c) The gases must be cooled before entering the oxidation chamber.
(d) In the catalyst chamber, once the reaction starts, the external source of heat is removed.
Q-4
State the effect of nitric acid on:
(a) Potassium iodide paper
(b) Heated copper
Q-5
Answer the questions
(a) With respect to the brown ring test for nitrates, explain:
(i) Freshly prepared ferrous sulphate is used.
(ii) The brown ring disappears if the test tube is disturbed.
(iii) Lead nitrate does not respond well to the brown ring test.
(b) Give the name and formula of the brown ring.
Q-6
Explain briefly:
(a) Nitric acid fumes in moist air
(b) Nitric acid stains the skin yellow.
Q-7
Give balanced equations for the action of heat on:
(a) Silver nitrate
(b) Potassium nitrate
(c) Copper(II) nitrate
(d) Ammonium nitrate
Chapter-11 Sulphuric Acid
Q-1
Answer the following Questions:
(a) Why is sulphuric acid called 'oil of vitriol'?
(b) Name the process by which sulphuric acid is manufactured on a large scale.
(c) Write the three main equations for the reactions involved in the above mentioned process.
(d) Name the most efficient catalyst that is used in this process.
(e) Why is it important that the gases are thoroughly purified before entering the catalyst chamber?
(f) Why is the sulphur trioxide absorbed in sulphuric acid and not in water?
(g) Explain why cone. sulphuric acid is diluted by adding the acid to the water and not vice versa.
(h) Sulphuric acid is a dibasic acid. Explain the term giving a suitable example.
(i) Name two other acids other than sulphuric acid which can be prepared by using sulphuric acid. Which property of sulphuric acid is used in preparing the named acids?
(j) Name the oxide of sulphur which reacts with water to give sulphuric acid.
(k) In the Contact Process, the direct reaction between the oxide of sulphur and water is avoided. In this process what does the oxide of sulphur react with instead of water and what is the name of the product?
(l) Give the name and formula of the acid salt which can give sodium ions and sulphate ions in solution.
Q-2
Give balanced equations to prepare sulphuric acid starting with each of the following substances.
(a) Ferrous sulphate crystals
(b) Sulphur (single step)
(c) Sulphur dioxide
(d) Sulphuryl chloride
Q-3
Sulphuric acid can be used in the laboratory to prepare the following substances:
(i) Hydrogen (ii) Carbon dioxide (iii) Carbon monoxide (iv) Sulphur dioxide (v) Hydrogen chloride (vi) Hydrogen sulphide (vii) Nitric acid
(a) Give a balanced equation for each of the above preparation.
(b) In which of the reactions would you use (give numbers only):
1 . Dilute sulphuric acid
2. Cone. sulphuric acid
(c) State the chemical property of sulphuric acid illustrated in each of the reactions in a.
Q-4
State what you would observe when cone. sulphuric acid is added to:
(a) Sugar crystals
(b) Copper sulphate crystals
Q-5
Distinguish between:
(a) Drying agent and dehydrating agent.
(b) Dilute sulphuric acid and cone. sulphuric acid.
Q-6
Sulphuric acid may act as each of the following:
A - an acid forming sulphates which are soluble in water
B - a compound forming sulphates which are insoluble in water
C - a dehydrating agent
D - a drying agent
E - an oxidising agent
Which one of the above properties A to E is shown by sulphuric acid when:
(a) Concentrated sulphuric acid is added to sugar?
(b) Dilute sulphuric acid reacts with lead nitrate solution?
(c) Dilute sulphuric acid is added to sodium hydroxide solution?
(d) Hydrogen sulphide gas is passed through concentrated sulphuric acid?
Q-7
Name of the gas evolved in each of the following cases:
(a) When zinc is treated with dilute sulphuric acid?
(b) When carbon is heated with cone. sulphuric acid?
Q-8
Write the equations for the laboratory preparation of the following salts using sulphuric acid:
(a) Iron (II) sulphate from iron
(b) Copper sulphate from copper
(c) Lead sulphate from lead nitrate
(d) Sodium sulphate from sodium carbonate
Q-9
Answer the following questions:
(a) Name the catalyst which helps in the conversion of sulphur dioxide to sulphur trioxide.
(b) In the Contact process for the manufacture of sulphuric acid, sulphur trioxide is not converted to sulphuric acid by reacting it with water. Instead a two-step procedure is used. Write the equations for the two steps involved.
(c) What type of substance will liberate sulphur dioxide from sodium sulphite.
(d) Write the equation for the reaction by which sulphur dioxide is converted to sodium sulphite.
(e) What is the purpose of the Contact Process?
(f) What two gases are combined during the Contact Process?
(g) Name the catalyst used in the process.
(h) Write the equation for the reaction between zinc and the final product of the Contact Process.
Q-10
Explain the following observations:
(a) When concentrated sulphuric acid is added to sugar, a black spongy mass is formed.
(b) Concentrated sulphuric acid is kept in airtight bottles.
(c) Whenever water is added to concentrated sulphuric acid or concentrated sulphuric acid is added to water, a large amount of heat is liberated. But the acid dangerously spurts out only when water is added to the concentrated acid.
Chapter-12 Organic Chemistry
Q-1
Answer the folllowing questions:
(a) What is organic chemistry?
(b) List five differences between organic compounds and inorganic compounds.
(c) Name three organic compounds of plant origin and three of animal origin.
(d) Give the general formula of alkanes. Write the name, structural formula and physical state of the compound containing :
(i) 3-carbon atoms
(ii) 8-carbon atoms
(e) Which of the compounds named in (d) would show:
(i) Isomerism
(ii) Higher melting point
(iii) Higher density
(iv) Lower boiling point
(f) List three characteristics of isomers.
(g) Write the structural formula of any two isomers of pentane, other than n-pentane.
(h) What is the main difference in the structural formula of the compounds n-pentane, iso-pentane, and neopentane?
Q-2
Briefly explain or define the following terms:
(a) Catenation
(b) Isomerism
(c) Homologous Series
Q-3
Write the formula and the IUPAC name of the following:
(a) Methane
(b) Ethane
(c) Methyl alcohol
(d) Ethyl alcohol
(e) Methyl chloride
(f) Ethyl chloride
Q-4
Fill in the blanks:
(a) Members of the alkene series containing ______ to ______ number of carbon atoms are liquids.
(b) Among isomers, the one with more branched chains has a ______ boiling point.
(c) The term ______ is used when one carbon atom is attached to four other carbon atoms.
(d) The molecular mass of any two homologues differ by ______ amu.
(e) The melting point of alkanes increases with ______ in molecular weight.
(f) The next higher homologue of pentane is _____ .
Q-5
What is meant by 'functional group'? Write the structure of the functional group that belongs to the following classes:
(a) Alcohol
(b) Aldehyde
(c) Carboxylic acid
Q-6
State two methods of preparing alkanes and use each of the stated methods to prepare an alkane that contains:
(a) 1 - carbon atom
(b) 3 - carbon atoms
Q-7
Answer the following questions:
(a) What is an alkyl group? Write the name and formula of the alkyl obtained from an alkane having 5 carbon atoms.
(b) Why is methane also known as 'marsh gas'?
(c) Show with the help of a neatly labelled diagram how methane can be prepared in the laboratory.
(d) How is methane gas collected in the laboratory? Why?
(e) What happens when methane is heated to a temperature of 1000°C in the absence of air? State one commercial use of the solid product formed in this reaction.
Q-8
Name two alkanes that exist as:
(a) Gases
(b) Liquids
(c) Solids
Q-9
Explain the following terms giving suitable examples:
(a) Saturated hydrocarbon
(b) Substitution reaction
(c) Cracking
Q-10
Give balanced equations for the following reactions. Also name the products formed in each reaction.
(a) Butane is heated in excess of air.
(b) Propane is heated in insufficient supply of air.
(c) Hexane is subjected to strong heat in the absence of air.
Q-11
Give equations to carry out the following conversions. Also state the necessary conditions for each conversion.
(a) Ethane ➔ Ethylene
(b) Sodium acetate ➔ Methane
(c) Methane ➔ Methanol
(d) Ethane ➔ Acetaldehyde
(e) Methane ➔ Methyl chloride
(f) Ethane ➔ Acetic acid
(g) Methane ➔ Carbon tetrachloride
(h) Methane ➔ Chloroform
Q-12
State two methods of preparing alkenes and use each of the stated methods to prepare an alkene that contains:
(a) 2-carbon atoms
(b) 4-carbon atoms
Q-13
Answer the following questions:
(a) Alkenes undergo addition reactions. Explain this property with the help of a suitable example.
(b) Give a reaction to obtain ethene by cracking. Also name the catalyst used.
(c) With the help of a neatly labelled diagram show how ethene is prepared in the laboratory.
(d) Write the molecular and structural formula of ethene.
Q-14
Write balanced equation for the following reactions. Also name the products formed.
(a) A mixture of ethene and hydrogen is passed over heated nickel.
(b) Ethene is treated with chlorine in the presence of sunlight.
(c) Ethene is treated with hydrochloric acid.
(d) Ethene is heated to 200°C under pressure.
(e) Ethene is burnt in air.
Q-15
Give balanced equation for the reaction of ethylene with each of the following:
(a) Oxygen
(b) Hydrogen
(c) Chlorine
(d) Bromine
Q-16
Answer the following Questions:
(a) Both, alkenes and alkynes are unsaturated hydrocarbons. State the one most significant difference between them.
(b) With the help of a neatly labelled diagram show how ethyne is prepared in the laboratory.
(c) Write the molecular and structural formula of ethyne.
(d) Explain why acetylene is a more reactive than ethene.
Q-17
Write balanced equation for the following reactions. Also name the products formed.
(a) A mixture of ethyne and hydrogen is passed over heated nickel.
(b) Ethyne is treated with chlorine.
(c) Ethyne is passed through bromine water.
Chapter-13 Practical Chemistry
Q-1
From the following list of substances, choose the one substance in each case which matches the description (i) to (vi) given below. (Write down the names exactly as they are given in the list. Do not write formulae)
Ammonium nitrate, calcium hydrogen carbonate, sodium hydrogen carbonate, lead carbonate, lead nitrate, potassium nitrate, sodium carbonate, copper carbonate, zinc carbonate
(a) A hydrogen carbonate which exists in the solid state.
(b) A carbonate not decomposed by heat.
(c) A green coloured carbonate which turns black on heating.
(d) A nitrate which gives off only oxygen when heated.
(e) A nitrate which on heating decomposes into dinitrogen oxide (nitrous oxide) and steam.
(f) A nitrate which gives off oxygen and nitrogen dioxide when heated.
Q-2
Copy and complete the following table which refers to the action of heat on some carbonates:

Q-3
Answer the following Questions:
(a) How will the action of dilute hydrochloride acid on sodium carbonate and sodium sulphite enable you to distinguish between these two compounds?
(b) Dilute hydrochloric acid and dilute sulphuric acid are both colourless solutions. How will the addition of barium chloride solution to each help to distinguish between the two?
Q-4
Choose the letters A, B, C or D to match the descriptions (i) to (vi) given below:
A Ammonia B Hydrogen chloride C Hydrogen sulphide b D Sulphur dioxide
(a) This gas can be oxidised to sulphur.
(b) This gas decolourises potassium permanganate solution.
(c) When this gas is bubbled through copper sulphate solution, a deep blue coloured solution is formed.
(d) This gas gives a white precipitate when reacted with silver nitrate solution acidified with dilute nitric acid.
(e) This gas burns in oxygen with a green flame.
(f) This gas can be obtained by the reaction between copper and concentrated sulphuric acid.
Q-5
The questions (i) to (v) refer to the following salt solutions listed A to F:
A Copper nitrate B lron(II) sulphate C lron(III) chloride
D Lead nitrate E Magnesium sulphate F Zinc chloride
(a) Which two solutions will give a white precipitate when treated with dilute Hydrochloric acid followed by Barium chloride solutions?
(b) Which two solutions will give a white precipitate when treated with dilute Nitric acid followed by Silver nitrate solutions?
(c) Which solution will give a white precipitate when either dilute Hydrochloric acid or dilute Sulphuric acid is added to it?
(d) Which solution becomes a deep/inky blue colour when excess of Ammonium hydroxide is added to it?
(e) Which solution gives a white precipitate with excess Ammonium hydroxide solution?
Q-6
Give one chemical test to distinguish between the following:
(a) Dilute hydrochloric acid and dilute nitric acid.
(b) Dilute hydrochloric acid and dilute sulphuric acid
Q-7
What is observed when:
(a) Hydrogen sulphide gas is passed through Lead acetate solution.
(b) Neutral litmus solution is added to sodium hydrogen carbonate solution.
(c) A small piece of iron is placed in copper sulphate solution.
(d) Ammonia gas is burnt in an atmosphere of oxygen in the absence of a catalyst.
(e) A glass rod dipped in ammonium hydroxide is brought near the mouth of the bottle containing concentrated hydrochloric acid.
(f) Excess ammonium hydroxide solution is added to lead nitrate solution.
(g) Bromine vapours are passed into a solution of ethyne in carbon tetrachloride.
(h) A zinc granule is added to copper sulphate solution.
(i) Zinc nitrate crystals are strongly heated.
(j) Sodium hydroxide solution is added to ferric chloride solution at first a little and then in excess.
Q-8
Identify the substance P, Q, R, Sand Tin each case based on the information given below:
(a) The deliquescent salt P, turns yellow on dissolving in water, and gives a reddish brown precipitate with a sodium hydroxide solution.
(b) The white crystalline solid Q is soluble in water. It liberates a pungent smelling gas when heated with sodium hydroxide solution.
(c) The pale green solid R turns reddish brown on heating. Its aqueous solution gives a white precipitate with barium chloride solution. The precipitate is insoluble in mineral acids.
(d) The reddish brown liquid S is dissolved in water. When Ethyne gas is passed through it, turns colourless.
(e) The nitrate T does not leave any residue on heating.
Q-9
The action of heat on the blue crystalline solid L gives a reddish brown gas M, a gas which re-lights a glowing splint and leaves a black residue.
When gas N, which has a rotten egg smell, is passed through a solution of La black precipitate is formed.
(a) Identify L, M and N (Name or formula).
(b) Write the equation for the action of heat on L.
(c) Write the equation for the reaction between the solution of Land the gas N.
Q-10
Match the following:
