Question bank
Chapter-1 The Language of Chemistry
Q-1
Answer the folliwing questions:
(a) What is meant by symbols of elements? Write the symbols of mercury, tungsten, bromine and lead.
(b) What is variable valency? How is the valency expressed according to the latest IUPAC system?
(c) What is the difference between Co and CO?
Q-2
Write the formula and valency of the following radicals:
(a) Ferric
(b) Ferricyanide
(c) Aluminate
(d) Sulphide
(e) Phosphide
(f) Phosphate
Q-3
Write the formulae of the following compounds:
(a) Potassium ferrocyanide
(b) Calcium hypochlorite
(c) Sodium thiosulphate
(d) Potassium permanganate
(e) Chromium sulphate
(f) Nickel nitrate
(g) Copper borate
(h) Sodium nitrate
(i) Calcium silicate
(j) Bismuth sulphate
Q-4
Write the following equations in symbols and balance them:
(a) Aluminium + sodium hydroxide + water ➔ Sodium aluminate + hydrogen
(b) Silver oxide + hydrogen peroxide ➔ Silver+ water+ oxygen
(c) Sodium carbonate + hydrochloric acid ➔ Sodium chloride + carbon dioxide + water
(d) Iron + sulphuric acid ➔ Iron(II) sulphate + hydrogen
(e) Ammonia + hydrochloric acid ➔ Ammonium chloride
(f) Copper + sulphuric acid ➔ Copper(II) sulphate dioxide + water
Q-5
Answer the following questions:
(a) Calculate the percentage of water in ferrous sulphate crystals. [Fe= 56, S =32, 0 = 16, H = 1]
(b) A 5.0 g sample of a hydrate of BaC12 was heated and only 4.3 g of the anhydrous salt remained. What percentage of water was in the hydrate?
Chapter-2 Chemical Reactions and Changes
Q-1
Define the following terms and answer the associated questions.
(a) Exothermic reaction : State the effect of an exothermic reaction on the surroundings. Also give an example of such a reaction.
(b) Endothermic reaction: State the effect of an endothermic reaction on the surroundings. Also give an example of such a reaction.
(c) Reversible reaction Give one example of a physical change that is reversible and one example of a chemical change that is reversible.
(d) Thermal Dissociation: Give two examples of a thermal dissociation reaction and two examples of a thermal decomposition reaction.
Q-2
Answer the following question:
(a) Give two examples, with balanced equations of reactions that occur in the presence of light.
(b) What causes chemical reactions to occur?
(c) List four ways by which the rate of a chemical reaction can be increased.
(d) Name and define the four important types of chemical reactions.
Q-3
Give balanced equations for the action of heat on the following compounds:
(a) Silver nitrate
(b) Zinc carbonate
(c) Sodium nitrate
(d) Red lead
(e) Copper(II) nitrate
(f) Silver oxide
(g) Magnesium hydroxide
(h) Lead(IV) oxide
(i) Calcium nitrate
(j) Ammonium chloride
(k) Lead(II) hydroxide
(l) Ferric hydroxide
(m) Aluminium carbonate
(n) Mercury(II) oxide
Q-4
Give a possible explanation for the following observations:
(a) When ammonium nitrate is dissolved in water contained in a beaker, the beaker becomes cold.
(b) When magnesium is dissolved in hydrochloric acid contained in a beaker, the beaker becomes hot.
(c) Gold is found free (uncombined) in nature.
(d) Compounds of sodium do not decompose on heating.
(e) Sea water is saline.
Q-5
Give balanced equations to prepare the following substances by using the method indicated alongside each.
(a) Potassium chloride : Simple displacement
(b) Iron[III] : chloride Synthesis
(c) Calcium chloride : Neutralisation
(d) Lead[II] chloride : Precipitation
(e) Potassium nitrite : Decomposition
Q-6
Name three insoluble:
(a) Metal chlorides
(b) Metal hydroxides
(c) Metal sulphates
(d) Metal carbonates
(e) Metal oxides
(f) Metal sulphides
Q-7
Name two soluble:
(a) Metal chlorides
(b) Metal hydroxides
(c) Metal sulphates
(d) Metal carbonates
(e) Metal oxides
(f) Metal sulphides
Q-8
Given below are four typical chemical properties of acids. Taking hydrochloric acid as an example, give a balanced equation to support each property.
(a) Acids react with bases to form salt and water.
(b) Acids react with carbonates to liberate carbon dioxide.
(c) Acids react with sulphites to liberate sulphur dioxide.
(d) Acids react with sulphides to liberate hydrogen sulphide.
Q-9
Name:
(a) The curtly white residue formed when silver nitrate is added to a salt containing the chloride radical.
(b) The insoluble white residue formed when sulphuric acid is added to a solution of barium chloride.
(c) The type ofreaction that occurs between an acid and a base to form salt and water.
Chapter-3 Water
Q-1
Define the following terms and answer the associated questions:
(a) Solute :In an alcohol and water mixture, can alcohol be considered the solute?
(b) Solvent :alcohol be considered the solute?
(c) Solution : Differentiate between a dilute solution and a concentrated solution.
(d) Solubility : Explain what is meant by the statement "Solubility of copper [II] sulphate is 16.2 g at 0°C" .
(e) Crystal : Give an example of a crystalline solid and state how it differs from an amorphous solid.
Q-2
Answer the following question:
(a) How is the solubility of a solid in liquid altered by:
(i) Temperature
(ii) Pressure
(b) How is the solubility of a gas in a liquid altered by:
(i) Temperature
(ii) Pressure
(c) What is a solubility curve? State three advantages of a solubility curve.
(d) Define "water of crystallisation".
(e) State three general observations when a coloured, hydrated salt like copper (II) sulphate is heated in a test-tube.
(f) Give the name and formula of a crystalline salt that:
(i) Does not contain any water of crystallisation
(ii) Contains 7 molecules of water
(iii) Contains 3 molecules of water
(iv) Contains 10 molecules of water
(v) Contains 2 molecules of water
(vi) Contains 5 molecules of water
(g) Name the ions common to all types of hard water.
(h) Name a process that removes only temporary hardness of water. Give a balanced equation to show the reaction taking place.
(i) Name a process that removes both, temporary and permanent hardness of water. Give a balanced equation to show the reaction.
(j) Explain, with the help of equations, why ordinary soap does not lather in hard water.
(k) State three advantages of hard water.
Q-3
Read the following passage carefully and point out at least three errors in it.
Green crystals of copper (II) sulphate are heated in a hard glass test tube. The crystals turn white, and a mist of colourless droplets collect on the cooler sides of the test-tube. When this liquid is allowed to trickle back to the white crystals (after cooling the test-tube) the crystals regain their original colour. The liquid also turns pink cobalt chloride paper blue.
Q-4
Explain the following terms:
(a) Unsaturated solution
(b) Saturated solution
(c) Supersaturated solution
(d) Crystallisation
Q-5
Oxygen is only slightly soluble in water. What would be the consequence if oxygen was:
(a) Completely insoluble in water
(b) Highly soluble in water?
Q-6
Give the name and formula of a salt, the solubility of which:
(a) Increases rapidly with increase in temperature.
(b) Increases gradually with increase in temperature.
(c) Increases very slightly with increase in temperature.
(d) Decreases with increase in temperature.
(e) Increases and then decreases, with increase in temperature.
Q-7
Which of the statements is true for hygroscopic substances?
(a) All are crystalline solids.
(b) They form saturated solution when exposed to moist air.
(c) They lose water on exposure to air.
(d) They may be liquids or crystalline solids.
Q-8
Differentiate between:
(a) Hard water and soft water.
(b) Temporary hardness and permanent hardness of water.
Q-9
Explain briefly:
(a) Rain water dissolves chalk hills.
(b) Hard water is not used for generating steam in boilers.
(c) The use of lead pipes for drinking water supply is being discontinued.
(d) A calculated amount of slaked lime is added to soften the hard water by Clarke's process.
Q-10
Carbon dioxide gas is passed through a calcium carbonate suspension in water. The resulting solution is divided into four parts.
(a) One sample is boiled.
(b) Another sample is mixed with soap solution and shaken.
(c) To the third sample, sodium carbonate solution is added.
(d) The fourth sample is made to react with slaked lime.
Chapter-4 Atomic Structure and Chemical Bonding
Q-1
Define the following terms and answer the associated questions:
(a) Atomic number : List 3 informations about the atom that is conveyed by the atomic number.
(b) Mass number : Why does the mass number exclude the mass of electrons ?
(c) Electronic configuration : State the maximum number of electrons that can be accommodated in the 5th shell of an atom.
(d) Valency : Name a metallic element that exhibits variable valency.
(e) Isotope : Name the isotope of hydrogen that contains 2 neutrons.
Q-2
Draw the atomic diagram of the following atoms:
(a) Helium p =2, E=2, N=2
(b) Argon : P = 18, E = 18, N=22
(c) Sulphur P= 16, E= 16, N= 16
(d) Calcium : P=20, E=20, N=20
Q-3
Answer the following Question:
(a) Name the elements that are represented by the following electronic configuration:
(i) 2, 4
(ii) 2, 6
(iii) 2
(iv) 2, 8, 6
(v) 2, 8, 8
(b) Write the electronic configuration of elements with the following atomic numbers:
(i) 20
(ii) 15
(iii) 9
(iv) 19
(v) 3
(c) The element lithium has atomic weight 6.9, atomic number of 3 and has isotopes of masses 6 and 7.
(i) Draw the atomic diagrams of the two isotopes.
(ii) Calculate the ratio in which the two isotopes exist.
(d) The elements P, Q, R and Shave atomic numbers 3, 4, 8 and 9 respectively. Classify the elements as metals and non-metals.
(e) The atom of an element has the 9 protons, 9 electrons and 10 neutrons.
(i) What is the atomic number of the element?
(ii) What is the mass number of the element?
(iii) Name the element.
(iv) Write the electronic configuration of the element.
(f) An element X has electronic configuration 2, 8, 2.
(i) Identify it as a metal, non-metal or inert gas.
(ii) Write the symbol of an ion of X. Also give a reason for its formation.
(g) Write the symbol of a particle X that contains 8 protons, 10 electrons and 8 neutrons.
(h) Write the electronic configuration of particle X.
(i) What would be the electronic configuration of the isotope of atom X which has atomic mass 18?
(j) Name the isotopes of hydrogen and give the structural diagram of each.
(k) XN is the metal nitride. Write:
(i) the ion of X
(ii) number of valence electrons in the atom of X.
(iii) its formula with phosphate, chromate, carbide
(l) Metal 'X' is divalent. With respect to 'X' answer the following:
(i) If the electrons are distributed in three shells, write the electronic configuration for 'X'.
(ii) If the number ofneutrons is 12, then find 'A' and 'Z'.
(iii) Frame the formula for the oxide of 'X'.
(m) An inert gas other than helium, has atomic number X. Write the symbol of the ion of the atom with atomic number X-2.
(n) The element lithium has atomic weight 6.9, atomic number of3 and has isotopes of masses 6 and 7. Draw the atomic diagrams of the two isotopes.
Q-4
Answer the following Question:
(a) What is a chemical bond? Name the three common types of chemical bonds.
(b) What is the cause of chemical reactivity between two elements?
(c) Describe how the transfer of electrons from sodium to chlorine takes place to form a molecule of sodium chloride
(d) Which type of elements form ionic compounds?
(e) What kind of bond is expected between carbon and chlorine?
(f) Give an example of a compound formed by these two elements.
(g) Write a property of the compound named in (f).
(h) What is an ion? State and explain the difference between an atom and the corresponding ion.
(i) Does the atomic number of an element change when its atoms form ions? Give reasons for your answer.
(j) How many electrons does oxygen need to achieve the electronic structure of neon atom?
(k) Justify the formula of magnesium oxide, MgO.
(l) What are the bond types present in each of the following? (i) Water
(ii) Nitrogen
(iii) Magnesium chloride
(iv) Calcium chloride
(m) State the meaning of octet configuration.
(n) Name two elements which do not acquire this configuration during bond formation.
(o) State the major difference between covalent and ionic bonds and give one example each of the covalent and ionic compounds.
(p) The elements W, X, Y, and Z have atomic numbers 7, 8, 9 and 11 respectively. Write the formulae of the compounds you would expect to form between the following pairs of elements and indicate the type of bonding present:
(i) Wand X
(ii) X and X
(iii) W and Z
(iv) Y and Y
(q) An element X has four electrons in the outermost shell of its atom and combines with another element Y having seven electrons in the outermost shell of its atom. The compound formed does not conduct electricity. What is the nature of the chemical bond in the compound?
(r) State one test by which sodium chloride can be distinguished from carbon tetrachloride.
Q-5
Differentiate between:
(a) Octet and Duplet
(b) Oxidation and Reduction on the basis of electron transfer.
(c) Cation and Anion
(d) Electrovalent bond and Covalent bond
Q-6
Explain briefly:
(a) An atom is electrically neutral.
(b) Non-metals form ions by gaining electrons while metals form ions by losing electrons.
(c) Isotopes exhibit same chemical properties.
(d) Isotopes differ in physical constants and weights.
(e) The atomic weight of chlorine is not a whole number.
(f) Covalent compounds are generally gases, liquids or soft solids.
(g) Ionic compounds are good conductors of electricity in their aqueous and molten state.
(h) Covalent compounds generally have a slow speed of chemical reaction.
Q-7
Name/State:
(a) The scientist who discovered the electron.
(b) The scientist who discovered the neutron.
(c) The scientist who first proposed the atomic theory.
(d) The scientist who discovered the atomic nucleus.
(e) A covalent hydride.
(f) An electrovalent hydride.
(g) The scientist who introduced the idea of atomic orbits and energy levels.
(h) The formula that determines the maximum capacity of a shell to accommodate electrons.
(i) The number of electrons in the outermost shell of radon ..
(j) The isotope of hydrogen that contains 1 neutron.
(k) The particle formed when an atom
a. loses electrons
b. gains electrons.
(l) The type of reaction that involves
a. loss of electrons.
b. gain of electrons.
(m) The type of bonding that involves
a. complete transfer of electrons.
b. mutual sharing of electron pairs.
(n) The number of paired electrons in a molecule of ammonia.
(o) The number of protons present in carbon - 14 (isotope).
Chapter-5 The Periodic Table
Q-1
Consider the following elements: Be, Li, Na, Ca, K. Which of these elements belong:
(i) to the same group?
(ii) to the same period?
Q-2
Choose the odd one in the following list of elements:
(i) F, Na, I, Br.
(ii) Na, K, Mg, Al, Si.
Q-3
Name two elements each of:
(i) Group 2
(ii) Group 13
(iii) Group 18
Q-4
An element has an atomic number 16. State:
(i) The period to which it belongs.
(ii) The number of valence electrons.
(iii) Whether it is a metal or non-metal.
Q-5
Answer the following question:
(a) What is an element?
(b) From the list given below, select those that represent elements. Sodiwn chloride, chlorine, blood, glucose, carbon dioxide, gold, steel, sodiwn
(c) What is the purpose of classifying elements?
(d) State the drawbacks of Dobereiner's system of classification.
(e) Mention the highlights ofNewlands system of classification.
(f) State the periodic law on which Mendeleev's periodic table was based.
(g) Mention three significant features of Mendeleev's Periodic Table, giving suitable examples.
(h) List three drawbacks of Mendeleev's Periodic Table.
(i) State the principle underlying the Modem Periodic Table.
(j) What is the significance of atomic nwnber in the classification of elements?
(k) What is meant by:
(i) A group
(ii) Period in a periodic table?
(l) Consider the following elements: Be, Li, Na, Ca, K. Which of these elements belong:
(i) to the same group?
(ii) to the same period?
(m) Choose the odd one in the following list of elements:
(i) F, Na, I, Br.
(ii) Na, K, Mg, Al, Si.
(n) Name two elements each of:
(i) Group 2
(ii) Group 13
(iii) Group 18
(o) Give the names and symbols of the third period elements of the long form periodic table.
(p) An element has an atomic number 16. State:
(i) The period to which it belongs.
(ii) The number of valence electrons.
(iii) Whether it is a metal or non-metal.
(q) Give the number of the group and the period, of the element having three shells with three electrons in valence shell.
Q-6
Write the correct alternative:
(a) The modem periodic table is divided into horizontal rows called ___________ . (groups/periods).
(b) The frrst period contains ______ (two/eight) elements.
(c) Elements of the second period are called ______ (bridge/transition) elements.
(d) The third period is a ______ (short/long) period
(e) The ______ (frrst/seventh) period is the shortest period.
(f) The fourth period contains ______ (18/32) elements.
(g) Elements of the __________ (second/third) period are typical elements.
(h) The ______ (fourth/fifth) period begins with rubidium and ends with xenon.
(i) The ____________ (second/third) period begins with lithiwn and ends with neon.
(j) The ________ (third/fourth) period begins with sodium and ends with argon.
Q-7
State briefly in what manner elements were classified by the following :
(a) Antoine Lavoisier
(b) John Dalton
(c) Dobereiner
(d) Newlands
Q-8
Rewrite the following statements after corrections, if necessary.
(a) Periods are the horizontal rows of elements.
(b) Isotopes are the elements of the same group.
(c) Elements in the same period have equal valencies.
(d) The metallic character of the elements in a period increases gradually on moving from left to right.
Q-9
Fill in the blanks based on the organisation of the Modern Periodic Table:
(a) The modem periodic table is divided into _______ columns called groups and _______ columns called periods.
(b) There are ________ main groups and ________ columns representing the various sub-groups.
(c) The group number is assigned to an element depending upon the number of ________ electrons.
(d) _______ metals are included in group IA.
(e) Alkaline earth metals are included in group ________ .
(f) Noble gases belong to the _______ group.
(g) _______ metals, the ________ series and the _______ series belong to group B.
(h) Halogen belong to group _______ .
(i) The reactivity of metals down group IA ________ down the group.
(j) The reactivity of halogens _______ down the group.
Q-10
The following question refers to the first three periods of the Periodic Table. Some of the elements are shown by letters. (The letters are not the symbols of the elements.)
(a) Which of the lettered elements is an inert gas?
(b) State two of the lettered elements which are in the same group.
(c) Give the name of each halogen element, together with the number of its position in the Table.
(d) Which one of the lettered elements would you expect to react most violently with chlorine? Explain.
(e) Which one of the lettered elements would you expect to form a compound of the compositionI g-atom of the element: 3 g-atoms of chlorine
(f) What would you expect the formula of a compound of hydrogen with the element I to be? (Use I as the symbol of the element.)
Q-11
In the Periodic Table given below, lithium, carbon, oxygen and neon are placed in their correct positions. The positions of nine other elements are represented by letters. These letters are not the symbols for the elements concerned.
By reference to the table, answer the following questions:
(i) Give the letter of the most reactive metal.
(ii) Give the letter of the most reactive non-metal.
(iii) Name the family of elements represented by L, Q, R, T.
(iv) Name one element in each case occurring in groups 2, 3 and 5.
Q-12
With reference to the above Table answer the following questions:
(i) What is the name given to the group to which T belongs?
(ii) Identify the most reactive element of group 1.
(iii) Identify the element from period 3 with least atomic size.
(iv) How many valence electrons are present in Q?
(v) Identify the noble gas of the fourth period.
(vi) In the compound between A and H what type of bond would be formed and give the molecular formula for the same.
Q-13
A group of elements in the Periodic Table are given below. Answer the following questions in relation to the above group of elements:
(Boron is the first member of the group and Thallium is the last). Boron, Aluminium, Gallium, Indium, Thallium
(a) Which element has the most metallic character?
(b) Which element would be expected to have the smallest atom?
(c) If the electronic configuration of Aluminium is 2, 8, 3, how many electrons are there in the outer shell of Thallium?
(d) The atomic number of Boron is 5. Write the chemical formula of the compound formed when Boron reacts with Chlorine.
(e) Will the elements in the group to the right of this Boron group be more metallic or less metallic in character? Justify your answer.
Q-14
Explain why:
(a) Atomic size increases moving down a group.
(b) Atomic size decreases moving across a period.
Chapter-6 Study of the first element - Hydrogen
Q-1
An experiment was set up as shown below to study a certain chemical property of hydrogen.
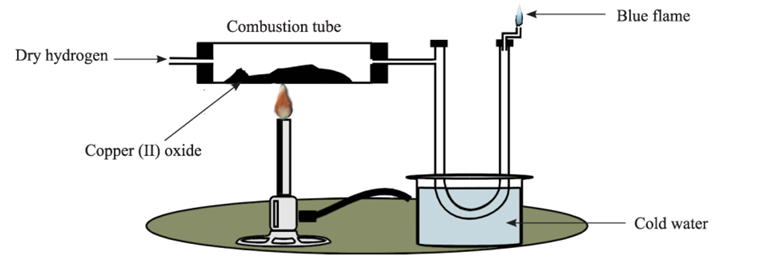
(i) It was observed that the heated black copper (II) oxide slowly turned pinkish red as dry hydrogen was passed over it. Name the pinkish red substance.
(ii) A colourless liquid collected in the 'U' tube. The liquid turned anhydrous white copper sulphate blue. Identify the liquid.
(iii) Write a balanced equation for the reaction between heated copper (II) oxide and dry hydrogen gas.
(iv) State and explain the property of hydrogen demonstrated by this experiment.
(v) Why is a blue flame observed if the gas coming out of the 'U' tube is ignited?
Q-2
Give balanced equations to prepare hydrogen starting with each of the following substances:
(a) A dilute mineral acid
(b) Cold water
(c) Acidulated water
(d) A hot concentrated alkali
(e) Steam
(f) A metal hydride
(g) Carbon (in the form of coke)
Q-3
You are provided with dilute hydrochloric acid and samples of the following metals:
Sodium, calcium, magnesium, iron, zinc, lead, copper and silver
Name:
(i) The metals that would not liberate hydrogen from the acid.
(ii) The metal(s) that would react explosively with the acid.
(iii) A metal that is above hydrogen in the activity series, yet does not displace hydrogen. Also give a reason for this behaviour.
(iv) A metal that liberates hydrogen very slowly from the acid.
(v) A metal that reacts moderately with the acid.
(b) If instead of dilute hydrochloric acid, you are provided with nitric acid, would hydrogen be liberated? Explain.
Q-4
In the laboratory preparation of hydrogen,
(a) How is the gas collected? Explain why this method is used.
(b) How is the gas dried?
(c) List three impurities that may be associated with the hydrogen and state how each impurity can be removed.
(d) List three precautions to ensure safe preparation and collection of the gas.
(e) How would you confirm that the gas collected is hydrogen?
Q-5
Answer the following Question:
(a) Name two large scale methods of obtaining hydrogen.
(b) What is water gas?
(c) How is water gas obtained?
(d) Name the products formed when steam is reacted with water gas at 1000°C in the presence of a catalyst. Also give a balanced equation for the reaction.
(e) Name the catalyst used in 5 (d).
(f) How are the products obtained in 5 (d) separated?
(g) Explain the following terms:
(i) Occlusion
(ii) Hydrogenation
(iii) Redox reaction
(h) Name a black reducing agent and a black oxidising agent.
Q-6
State one property of hydrogen:
(a) That made it useful in meteorological balloons.
(b) Due to which it is no longer used in meteorological balloons.
(c) Due to which it is used in metallurgy.
(d) That makes it a good fuel.
Q-7
Hydrogen can be obtained by various methods. Give a balanced equation to prepare hydrogen by each of the methods stated below.
(a) Action of cold water on a reactive metal.
(b) Action of hot cone.alkali on an amphoteric metal.
(c) Action of steam on a weakly electropositive metal.
(d) Action of water on a metal hydride.
(e) Action of steam (excess) on a non-metal.
(f) Action of steam on water gas.
(g) Passage of an electric current through acidulated water.
(h) Passage of an electric current through acidulated water.
Q-8
State what you would observe when:
(a) A piece of sodium is added to a trough containing cold water to which a few drops of red litmus solution has been added.
(b) A piece of potassium is added to a trough containing dilute hydrochloric acid.
(c) Dilute hydrochloric acid is added to copper turnings.
(d) Dilute nitric acid is added to zinc granules.
Q-9
Fill in the blanks with respect to the physical properties of hydrogen.
(a) Hydrogen is ____ times lighter than air and conducts heat ____ times better than air.
(b) One litre of hydrogen weighs ____ g at STP.
(c) Only ____ cm^3 of hydrogen gas dissolves in 100 cm^3 of water under ordinary conditions.
(d) Hydrogen liquefies below _____ °Cat a pressure of 20 atm.
(e) Liquid hydrogen is ____ and boils at ____ °C.
Q-10
An experiment was set up as shown below to study a certain chemical property of hydrogen
(a) It was observed that the heated black copper (II) oxide slowly turned pinkish red as dry hydrogen was passed over it. Name the pinkish red substance.
(b) A colourless liquid collected in the 'U' tube. The liquid turned anhydrous white copper sulphate blue. Identify the liquid.
(c) Write a balanced equation for the reaction between heated copper (II) oxide and dry hydrogen gas.
(d) State and explain the property of hydrogen demonstrated by this experiment.
(e) Why is a blue flame observed if the gas coming out of the 'U' tube is ignited?
Q-11
Name:
(a) The residue formed when steam is passed over heated iron.
(b) The gas that has replaced hydrogen in meteorological balloons.
(c) A metal that yields hydrogen when reacted with an acid as well as an alkali.
(d) A metal that can liberate hydrogen from steam but not from boiling water.
(e) A metal that reacts reversibly with steam.
(f) Two fuel gases in which hydrogen forms a major component.
(g) The process by which oil is converted into fat in the presence of a catalyst.
(h) The process in which iron (III) oxide is used as a catalyst.
(i) The compound formed when sodium combines with hydrogen.
(j) The product formed (along with hydrogen) when aluminium reacts with caustic soda.
(k) Two metals that can be reduced by hydrogen.
(l) Two metal oxides that cannot be reduced by hydrogen.
(m) Two oxidising agents that contain hydrogen.
(n) Two reducing agents that contain oxygen.
(o) An oxidising agent that does not contain oxygen.
Chapter-7 Study of Gas Laws
Q-1
Answer the Following Questions:
(i) Define Boyle's Law.
(ii) When air is filled into a balloon, the pressure inside the balloon increases. So does the volume of the balloon. Explain this apparent contradiction of Boyle's law.
(iii) In a vessel containing water, air bubbles at the bottom of the vessel are seen to increase in volume as they rise to the top of the vessel. Explain this observation.
(vi) A gas at constant temperature and having an initial volume of 600 mL is allowed to expand until it attains a final volume of 2400 mL. If the gas was originally at a pressure of 2 atmospheres, find the new pressure acting on the gas.
(v) A 2 litre container of nitrogen had a pressure of 3.2 atm. What volume would be necessary to decrease the pressure to 1 atm. at the same temperature?
(vi) Ammonia gas occupies a volume of 450 mL at a pressure of720 mm of Hg. What volume will it occupy at standard pressure at the same temperature?
(vii) A certain gas occupies a volume of 1500 cm^3 at a certain pressure. When the pressure was altered to 5 atm, the volume occupied by the gas was 3000 cm^3 Assuming the temperature to be constant, find the initial pressure of the gas.
(viii) Define Charles' Law.
(ix) A gas occupies 200 cm^3 at 27 °c. What is its volume at 51 °c, the pressure being kept constant?
(x) A gas has a volume of 380 cm^3 at 91°c and 72 mm of Hg pressure. What is its volume:
(i) at STP?
(ii) at 0°C and 70 cm of Hg pressure?
(xi) A sample of argon gas is cooled and its volume has changed from 380 mL to 250 mL. If its final temperature was -55°c, what was its original temperature? [Pressure constant]
(xii) A gas occupies 150 cm3 at 57cc. Assuming that there is no change in pressure, find the temperature to which the gas must be heated so that the volume triples.
(xiii) At 27°c a gas is compressed to half of its volume. To what temperature must it now be heated so that it occupies its original volume?
(xiv) At constant pressure, a gas at -33°c is heated to 127°c. Find the percentage increase in the volume of the gas.
(xv) The volume of a certain amount of a gas at 27°c and 760 mm pressure is 100 cm^3 Calculate its volume at 327 °c and 1520 mm pressure.
(xvi) The volume of a certain gas at constant temperature was found to be 1400 cm^3 when the pressure was 1200 mm. If the pressure is decreased by 30%, find the new volume.
(xvii) A gas cylinder containing cooking gas can withstand a pressure of 14.9 atmosphere. The pressure gauge of the cylinder indicates 12 atmosphere at 27°C. Due to a sudden fire in the building, its temperature starts rising. At what temperature will the cylinder explode?
(xviii) A fixed volume of a gas occupies 760 cm^3 at 27°C and 70 cm of Hg. What will be its volume at STP?
(xix) At 0°C and 760 mm Hg pressure, a gas occupies a volume of 100 cm3 The Kelvin temperature (Absolute temperature) of the gas is increased by one-fifth while the pressure is increased one and a halftimes. Calculate the final volume of the gas.
(xx) Carbon dioxide occupies a volume of 512 cm^3 at STP. Find its volume at 27°C and 720 mm of Hg.
(xxi) The volume of a certain gas at constant temperature was 1200 cm^3 when the pressure was 840 mm. If the volume is decreased to 60% of the original volume, what is the change in pressure?
(xxii) A gas 'X' is contained in a vessel of capacity 10 litres and subjected to a pressure of 20 atm. If this container is connected to another empty container of similar capacity, what will be the pressure acting on gas 'X' in the combined container? (temperature is constant)
(xxiii) A gas occupies a volume of 1600 cm^3 at a pressure 'y'. If the pressure is changed to 5 atm, the volume of the gas increases to 1800 cm^3 Find the value of 'y'.
(xxiv) A gas at constant pressure occupies a volume of 600 mL. On heating to 80°C it expands to occupy a volume of 2400 mL. Find the original temperature of the gas.
(xxv) To what temperature must a gas at 27°C be cooled in order to reduce its volume to 1/3 its original volume, pressure remaining constant.
(xxvi) A gas occupies a volume of 20 litres at 27°C and 70 cm of pressure. Calculate its volume at STP.
(xxvii) When a certain gas is heated, its volume increases by 50% and pressure decreases to 75% of its original value. If its original temperature was -15°C, find the temperature.
(xxviii) Ammonia gas occupies a volume of 450 mL at a pressure of720 mm of Hg. What volume will it occupy at standard pressure at the same temperature?
(xxix) A toy balloon has a volume of 1450 mL when it is filled with helium at a temperature of 17°C and a pressure of 750 mm of Hg. It rises to an altitude where the temperature falls to -23°C and pressure falls to 40% of the original. What is its volume?
(xxx) A certain quantity of gas at normal temperature is placed in a closed vessel which has a volume of 2.73 litres.
a. At what temperature will the volume of the gas be 2000 mL, pressure remaining constant?
b. At what temperature will the volume be 3 litres?
(xxxi) 546 mL of a certain gas at 0°C is taken in a closed vessel.
a. What will its volume be if the temperature is raised to 10°C?
b. How much space will this gas occupy at -10°C? (pressure is constant in both)
(xxxii) 50 mL of hydrogen is collected over water at 17°C and 750 mm of pressure. Calculate the volume of dry gas at STP. The vapour pressure of dry gas at 17°C is 14 mm of Hg.
Q-2
Explain briefly:
(a) The Kelvin scale has been adopted for chemical calculation.
(b) The Absolute zero is a theoretical concept.
(c) When stating the volume of a gas, the pressure and temperature should also be given.
Q-3
Point out the law illustrated in the following examples:
(a) Bread rises while baking because carbon dioxide is given off when yeast is heated.
(b) Car tyres expand on a hot day.
(c) A balloon will burst if you sit on it.
Q-4
A fully inflated toy balloon containing 300 cm^3 of air at room temperature (27°C) is placed in a bucket of ice.
(i) a. State briefly what you would observe.
b. Give a reason to support your observation.
c. Calculate the volume occupied by air when the balloon is placed in ice.
Chapter-8 Atmospheric Pollution
Q-1
Answer the following questions:
(a) What is acid rain? How is it formed?
(b) Name the natural and man made sources of acid rain.
(c) Name the gases responsible for acid rain.
(d) What are the remedial measures to control acid rain?
(e) What is the greenhouse effect? How does it lead to global warming?
(f) Name the major greenhouse gases and their sources.
(g) State four consequences of the greenhouse effect on the environment.
(h) Suggest three methods to control the emission of greenhouse gases.
(i) When was the Ozone hole discovered? How is ozone useful in the stratosphere?
(j) How is ozone formed in the stratosphere?
(k) Name four ozone depleting substances.
(l) What are Chlorofluorocarbons? Name the general sources of chlorofluorocarbons.
Q-2
Explain with balanced equations how:
(a) Nitric acid is formed when internal combustion takes place in the engines of automobiles.
(b) Sulphuric acid is formed when fossil fuels are burnt.
Q-3
Explain the terms:
(a) Stone cancer
(b) Chlorosis
(c) Necrosis
Q-4
Explain what is meant by:
(a) Coral reef bleaching
(b) Plant and animal range shifts
Q-5
List the harmful effects of ozone depletion on the following:
(a) Human health
(b) Aquatic ecosystems
(c) Biogeochemical cycles
Q-6
Answer the following questions:
(a) What general trend in ozone concentration is shown on the graph in Figure - B? How does the data for the years 1977-1987 on this graph compare to the same time span on the graph in Figure-A? State your observation.
Chapter-9 Practical Chemistry
Q-1
Name:
(a) A blue-green amorphous compound which leaves a black residue on heating.
(b) A white solid that decripitates on heating.
(c) A red-brown gas that turns potassium iodide paper brown.
(d) A gas that turns limewater milky.
(e) A solid that is rust when hot, yellow when cold and fuses with glass on heating in a test-tube.
(f) A glowing incense stick glows even more brightly when introduced into a jar containing this gas.
(g) This gas extinguishes a glowing splinter.
(h) The presence of this cation in a compound produces a brick flame when flame test is performed.
(i) On heating, this compound leaves a residue which is yellow when hot and white when cold; and a gas which is colourless, odourless and turns moist blue litmus red.
(j) A gas evolved when ferrous sulphate crystals are heated strongly in a test-tube.
Q-2
Select from the list below the gas that matches the description given in each case and answer the questions that follow: Ammonia chlorine hydrogen chloride sulphur dioxide
(a) Gas A is a reducing agent which contains oxygen.
(i) What is the name of gas A?
(ii) What would you observe if gas A is bubbled through acidified potassium dichromate solution?
(b) Gas B turns moist red litmus paper blue.
(i) What property of gas B is evident from the litmus test?
(ii) What is the name of gas B?
(c) When gas C is mixed with gas B, dense white fumes are seen and there is no other product.
(i) What is the name of gas C?
(ii) Name of the product of the reaction between gas B and gas C.
(iii) Name the type ofreaction that takes place between gas B and gas C.
(d) Gas D bleaches moist litmus paper.
(i) What property of gas D is evident from the litmus test?
(ii) What is the name of gas D?
Q-3
Identify the colours of the following:
(a) Colour imparted to flame by washing soda.
(b) Colour of residue when an orange crystalline salt is heated.
(c) Colour of vapours evolved when iodine is heated.
(d) Colour of the gas evolved when concentrated sulphuric acid is added to zinc sulphide.
Q-4
When concentrated sulphuric acid is added to a white crystalline salt (X), a colourless gas is evolved which fumes in moist air. On performing the flame test, a lilac flame is observed.
(a) Name the gas evolved and give a test to identify it.
(b) Name the metal ion present in the compound X.
(c) Write the name and formula of salt X
Q-5
X, Y and Z are crystalline, water soluble solids which have a common anion. To help you to identify X, Y and Z, you are provided with the following experimental observations. Complete the corresponding inferences in (i) to (iv).
(a) A reddish-brown gas is obtained when X, Y and Z are separately warmed with concentrated sulphuric acid and copper turnings added to the mixture.
Inference 1: The common anion is the _______ ion.
(b) When X is heated, it melts and gives off only one gas which relights a glowing splint.
Inference 2: The cation in Xis either _______ or _______ .
(c) The action of heat on Yproduces a reddish-brown gas and a yellow residue which fuses with the glass of the test-tube.
Inference 3: The metal ion present in Y is the _______ ion.
(d) When Z is heated it leaves no residue. Warming Z with sodium hydroxide solution liberates a gas which turns moist red litmus paper blue.
Inference 4: Z contains the _______ cation.
(v) Write the equations for the following reactions:
a. X and concentrated sulphuric acid. [One equation only for either of the cations given in inference (ii)]
b. Action of heat on Y.